当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fabricating efficient CdSe–CdS photocatalyst systems by spatially resetting water splitting sites
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7ta06085h Zhijian Wang 1, 2, 3, 4, 5 , Junmei Wang 1, 2, 3, 4, 5 , Li Li 1, 2, 3, 4, 5 , Jianfeng Zheng 1, 2, 3, 4, 5 , Suping Jia 1, 2, 3, 4, 5 , Jiazang Chen 1, 2, 3, 4, 5 , Bin Liu 6, 7, 8 , Zhenping Zhu 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7ta06085h Zhijian Wang 1, 2, 3, 4, 5 , Junmei Wang 1, 2, 3, 4, 5 , Li Li 1, 2, 3, 4, 5 , Jianfeng Zheng 1, 2, 3, 4, 5 , Suping Jia 1, 2, 3, 4, 5 , Jiazang Chen 1, 2, 3, 4, 5 , Bin Liu 6, 7, 8 , Zhenping Zhu 1, 2, 3, 4, 5
Affiliation
|
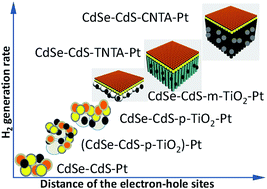
Water oxidation and reduction over semiconductor-based photocatalysts intrinsically occur at different spatial sites. Modulation of the reaction sites and charge transfer between them are logically important in speeding up the reaction. Here, we demonstrate that divorcing a CdSe–CdS–Pt donor–acceptor system on different surface sites of TiO2 can significantly increase the H2 generation rate. The increase is derived from an effective reset of water oxidation and reduction sites. Widening of the site distance by employing a TiO2 membraniform acceptor effectively decreases the electron–hole recombination especially for a membrane of TiO2 nanotube arrays. The oriented charge transfer characteristic of TiO2 nanotube arrays benefits long-range electron transport, thereby increasing the electron lifetime and reaction rate. When more conductive carbon nanotube arrays serve as an electron acceptor to replace TiO2, the electron transport is greatly improved, resulting in an ultrahigh H2 generation rate of 1270 mmol g−1 h−1. This work provides a basis for the design and construction of highly efficient photocatalysts through rational modulation of reaction sites and charge transport.
中文翻译:

通过在空间上重置水分解位来制造有效的CdSe–CdS光催化剂系统
水的氧化和基于半导体的光催化剂的还原本质上发生在不同的空间位置。在加速反应中,对反应位点的调节以及它们之间的电荷转移在逻辑上很重要。在这里,我们证明了在TiO 2的不同表面上离解CdSe–CdS–Pt供体–受体系统可以显着提高H 2生成速率。增加是由于水氧化和还原位点的有效复位而引起的。通过使用TiO 2膜状受体扩大位点距离可以有效地减少电子-空穴复合,尤其是对于TiO 2纳米管阵列的膜而言。TiO 2的定向电荷转移特性纳米管阵列有利于远距离电子传输,从而增加了电子寿命和反应速率。当更多导电的碳纳米管阵列用作电子受体来代替TiO 2时,电子传输得到极大改善,导致H 2的产生速率超高,为1270 mmol g -1 h -1。这项工作通过合理调节反应位点和电荷传输为高效光催化剂的设计和构建提供了基础。
更新日期:2017-09-25
中文翻译:

通过在空间上重置水分解位来制造有效的CdSe–CdS光催化剂系统
水的氧化和基于半导体的光催化剂的还原本质上发生在不同的空间位置。在加速反应中,对反应位点的调节以及它们之间的电荷转移在逻辑上很重要。在这里,我们证明了在TiO 2的不同表面上离解CdSe–CdS–Pt供体–受体系统可以显着提高H 2生成速率。增加是由于水氧化和还原位点的有效复位而引起的。通过使用TiO 2膜状受体扩大位点距离可以有效地减少电子-空穴复合,尤其是对于TiO 2纳米管阵列的膜而言。TiO 2的定向电荷转移特性纳米管阵列有利于远距离电子传输,从而增加了电子寿命和反应速率。当更多导电的碳纳米管阵列用作电子受体来代替TiO 2时,电子传输得到极大改善,导致H 2的产生速率超高,为1270 mmol g -1 h -1。这项工作通过合理调节反应位点和电荷传输为高效光催化剂的设计和构建提供了基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号