当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Applications of Iridium-Catalyzed Asymmetric Allylic Substitution Reactions in Target-Oriented Synthesis
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.accounts.7b00300 Jianping Qu 1 , Günter Helmchen 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.accounts.7b00300 Jianping Qu 1 , Günter Helmchen 2
Affiliation
|
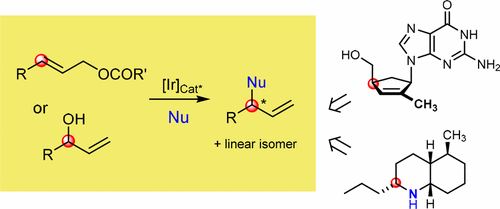
Metal catalyzed allylic substitution is a cornerstone of organometallic and synthetic chemistry. Enantioselective versions have been developed with catalysts derived from transition metals, most notably molybdenum, nickel, ruthenium, rhodium, iridium, palladium, and copper. The palladium- and the iridium-catalyzed versions have turned out to be particularly versatile in organic synthesis because of the very broad scope of the nucleophile and great functional group compatibility. Assets of the iridium-catalyzed reaction are the formation of branched, chiral products from simple monosubstituted allylic substrates, high degrees of regio- and enantioselectivity, and use of modular, readily available chiral ligands. The possibility to use carbon, nitrogen, oxygen, and sulfur compounds as well as fluoride as nucleophiles allows a wide range of chiral building blocks to be prepared.
中文翻译:

铱催化的不对称烯丙基取代反应在目标合成中的应用
金属催化的烯丙基取代是有机金属和合成化学的基础。用衍生自过渡金属,最著名的是钼,镍,钌,铑,铱,钯和铜的催化剂开发了对映选择型。由于亲核试剂的范围很广并且官能团的相容性很高,因此钯和铱催化的化合物在有机合成中特别有用。铱催化反应的资产是由简单的单取代烯丙基底物形成支链手性产物,高度的区域选择性和对映选择性以及使用模块化的,易于获得的手性配体。使用碳,氮,氧的可能性,
更新日期:2017-09-22
中文翻译:

铱催化的不对称烯丙基取代反应在目标合成中的应用
金属催化的烯丙基取代是有机金属和合成化学的基础。用衍生自过渡金属,最著名的是钼,镍,钌,铑,铱,钯和铜的催化剂开发了对映选择型。由于亲核试剂的范围很广并且官能团的相容性很高,因此钯和铱催化的化合物在有机合成中特别有用。铱催化反应的资产是由简单的单取代烯丙基底物形成支链手性产物,高度的区域选择性和对映选择性以及使用模块化的,易于获得的手性配体。使用碳,氮,氧的可能性,


























 京公网安备 11010802027423号
京公网安备 11010802027423号