当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of Bis(μ3-silylyne) Complexes via Consecutive Si–H Bond Cleavage at a Triruthenium Site
Organometallics ( IF 2.8 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.organomet.7b00548 Hiroyuki Tsuruda 1 , Yoshihiro Hayashi 1 , Susumu Kawauchi 1 , Toshiro Takao 1, 2
Organometallics ( IF 2.8 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.organomet.7b00548 Hiroyuki Tsuruda 1 , Yoshihiro Hayashi 1 , Susumu Kawauchi 1 , Toshiro Takao 1, 2
Affiliation
|
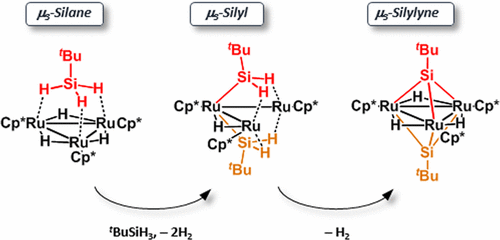
The reaction of the μ3-silane complex {Cp*Ru(μ-H)}3(μ3-η2:η2:η2-H3SitBu) (2) with tBuSiH3 caused η2-Si–H bond scissions and yielded the bis(μ3-silyl) complex (Cp*Ru)3(μ3-η2:η2-H2SitBu)2(μ-H) (4). The structure of 4, which adopts an open Ru3 framework, is characteristic of trinuclear complexes adopting a 50-electron configuration and was determined by X-ray diffractometry (XRD). Thermolysis of 4 resulted in further dehydrogenation and afforded a bis(μ3-silylyne) complex, {Cp*Ru(μ-H)}3(μ3-SitBu)2 (5a), whose highly symmetrical structure was unambiguously determined by XRD. A phenylsilylyne analogue, 5b, was also obtained by the reaction of {Cp*Ru(μ-H)}3(μ3-η2:η2-H2SiPh)(H) (3b) with PhSiH3 but via a different intermediate, the μ3-silylene−μ-silylene complex {Cp*Ru(μ-H)}3(μ3-η2-HSiPh)(μ-SiHPh) (6). The difference in intermediate was rationalized as arising from the steric repulsion between the substituent at the silicon atom and the surrounding Cp* groups. That is, the bulky tBu group retards the formation of a Ru–Si σ bond. In fact, μ3-silyl complex 3c was exclusively obtained by the reaction of {Cp*Ru(μ-H)}3(μ3-H)2 (1) with nBuSiH3, whereas its propensity toward oxidative addition is similar to that of tBuSiH3. The series of μ3-silane, μ3-silyl, μ3-silylene, and μ3-silylyne complexes could be a model for successive σ-bond cleavage at a trinuclear site.
中文翻译:

通过在三钌位点的连续Si-H键裂解制备Bis(μ3-甲硅烷基)配合物
所述μ的反应3 -硅烷复杂{的Cp *茹(μ-H)} 3(μ 3 -η 2:η 2:η 2 -H 3的Si吨卜)(2)配有吨BuSiH 3引起的η 2 - Si-H键和链断裂,得到双(μ 3 -甲硅烷基)络合物(CP * Ru)的3(μ 3 -η 2:η 2 -H 2的Si吨丁基)2(μ-H)(4)。4的结构,它采用开放式Ru 3构架是采用50电子构型的三核配合物的特征,并由X射线衍射法(XRD)确定。热解的4导致进一步脱氢时,可以得到双(μ 3 -silylyne)络合物,{的Cp *茹(μ-H)} 3(μ 3 -Si吨丁基)2(图5a),其高度对称的结构明确地确定由XRD。甲phenylsilylyne类似物,5B,还通过反应得到{的Cp *茹(μ-H)} 3(μ 3 -η 2:η 2 -H 2 SIPH)(H)(图3b)与PhSiH 3但是经由不同的中间时,μ 3 -silylene-μ-亚甲硅烷复合{的Cp *茹(μ-H)} 3(μ 3 -η 2 -HSiPh)(μ-SiHPh)(6)。中间体之间的差异被合理化,是由于硅原子上的取代基与周围的Cp *基团之间发生空间排斥而引起的。也就是说,庞大的t Bu基团阻碍了Ru-Siσ键的形成。事实上,μ 3 -甲硅烷复合3C是专门由{的Cp *茹(μ-H)}反应得到3(μ 3 -H)2(1)配有Ñ BuSiH 3,而其氧化加成倾向与t BuSiH 3相似。该系列μ的3 -硅烷,μ 3 -甲硅烷,μ 3 -silylene,μ 3个-silylyne络合物可能是在一个三核位点用于连续σ键裂解的模型。
更新日期:2017-09-23
中文翻译:

通过在三钌位点的连续Si-H键裂解制备Bis(μ3-甲硅烷基)配合物
所述μ的反应3 -硅烷复杂{的Cp *茹(μ-H)} 3(μ 3 -η 2:η 2:η 2 -H 3的Si吨卜)(2)配有吨BuSiH 3引起的η 2 - Si-H键和链断裂,得到双(μ 3 -甲硅烷基)络合物(CP * Ru)的3(μ 3 -η 2:η 2 -H 2的Si吨丁基)2(μ-H)(4)。4的结构,它采用开放式Ru 3构架是采用50电子构型的三核配合物的特征,并由X射线衍射法(XRD)确定。热解的4导致进一步脱氢时,可以得到双(μ 3 -silylyne)络合物,{的Cp *茹(μ-H)} 3(μ 3 -Si吨丁基)2(图5a),其高度对称的结构明确地确定由XRD。甲phenylsilylyne类似物,5B,还通过反应得到{的Cp *茹(μ-H)} 3(μ 3 -η 2:η 2 -H 2 SIPH)(H)(图3b)与PhSiH 3但是经由不同的中间时,μ 3 -silylene-μ-亚甲硅烷复合{的Cp *茹(μ-H)} 3(μ 3 -η 2 -HSiPh)(μ-SiHPh)(6)。中间体之间的差异被合理化,是由于硅原子上的取代基与周围的Cp *基团之间发生空间排斥而引起的。也就是说,庞大的t Bu基团阻碍了Ru-Siσ键的形成。事实上,μ 3 -甲硅烷复合3C是专门由{的Cp *茹(μ-H)}反应得到3(μ 3 -H)2(1)配有Ñ BuSiH 3,而其氧化加成倾向与t BuSiH 3相似。该系列μ的3 -硅烷,μ 3 -甲硅烷,μ 3 -silylene,μ 3个-silylyne络合物可能是在一个三核位点用于连续σ键裂解的模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号