当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Orthogonal Tyrosine and Cysteine Site-Directed Spin Labeling for Dipolar Pulse EPR Spectroscopy on Proteins
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.jpclett.7b02220 Christoph Gmeiner 1 , Daniel Klose 1 , Elisabetta Mileo 2 , Valérie Belle 2 , Sylvain R. A. Marque 3, 4 , Georg Dorn 5 , Frédéric H. T. Allain 5 , Bruno Guigliarelli 2 , Gunnar Jeschke 1 , Maxim Yulikov 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.jpclett.7b02220 Christoph Gmeiner 1 , Daniel Klose 1 , Elisabetta Mileo 2 , Valérie Belle 2 , Sylvain R. A. Marque 3, 4 , Georg Dorn 5 , Frédéric H. T. Allain 5 , Bruno Guigliarelli 2 , Gunnar Jeschke 1 , Maxim Yulikov 1
Affiliation
|
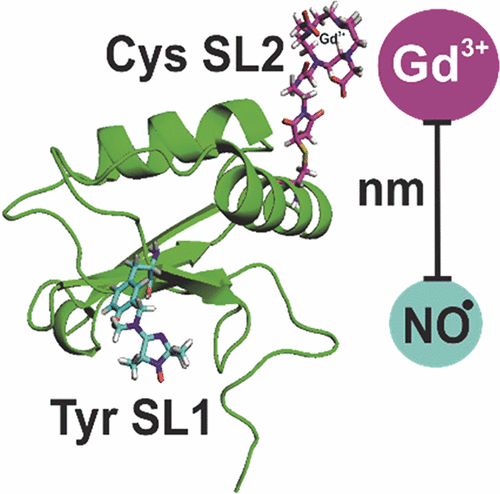
Site-directed spin labeling of native tyrosine residues in isolated domains of the protein PTBP1, using a Mannich-type reaction, was combined with conventional spin labeling of cysteine residues. Double electron–electron resonance (DEER) EPR measurements were performed for both the nitroxide–nitroxide and Gd(III)–nitroxide label combinations within the same protein molecule. For the prediction of distance distributions from a structure model, rotamer libraries were generated for the two linker forms of the tyrosine-reactive isoindoline-based nitroxide radical Nox. Only moderate differences exist between the spatial spin distributions for the two linker forms of Nox. This strongly simplifies DEER data analysis, in particular, if only mean distances need to be predicted.
中文翻译:

正交酪氨酸和半胱氨酸定点自旋标记用于蛋白质的偶极脉冲EPR光谱
使用曼尼希型反应,将蛋白质PTBP1分离域中天然酪氨酸残基的定点旋转标记与半胱氨酸残基的常规旋转标记结合在一起。对同一蛋白质分子中的氮氧化物-硝基氧化物和Gd(III)-硝基氧化物标记组合进行了双电子电子共振(DEER)EPR测量。为了从结构模型预测距离分布,生成了酪氨酸反应性基于异吲哚啉的氮氧化物自由基Nox的两种接头形式的旋转异构体文库。Nox的两种接头形式的空间自旋分布之间仅存在适度差异。特别是在仅需要预测平均距离的情况下,这极大地简化了DEER数据分析。
更新日期:2017-09-23
中文翻译:

正交酪氨酸和半胱氨酸定点自旋标记用于蛋白质的偶极脉冲EPR光谱
使用曼尼希型反应,将蛋白质PTBP1分离域中天然酪氨酸残基的定点旋转标记与半胱氨酸残基的常规旋转标记结合在一起。对同一蛋白质分子中的氮氧化物-硝基氧化物和Gd(III)-硝基氧化物标记组合进行了双电子电子共振(DEER)EPR测量。为了从结构模型预测距离分布,生成了酪氨酸反应性基于异吲哚啉的氮氧化物自由基Nox的两种接头形式的旋转异构体文库。Nox的两种接头形式的空间自旋分布之间仅存在适度差异。特别是在仅需要预测平均距离的情况下,这极大地简化了DEER数据分析。


























 京公网安备 11010802027423号
京公网安备 11010802027423号