当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Cobalt Speciation on γ-Al2O3 in the Presence of Carboxylate Ligands in Supported Catalyst Preparation
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.jpcc.7b06559 A. Davantès 1 , C. Schlaup 2 , X. Carrier 2 , M. Rivallan 3 , G. Lefèvre 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.jpcc.7b06559 A. Davantès 1 , C. Schlaup 2 , X. Carrier 2 , M. Rivallan 3 , G. Lefèvre 1
Affiliation
|
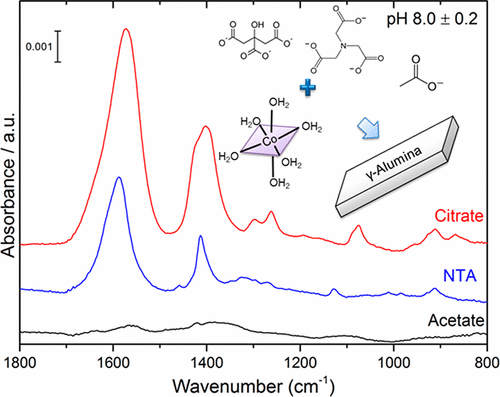
Molecular-scale understanding of metal impregnation on a porous oxide support is a key step toward the development of optimized (and cost-effective) catalytic materials with enhanced activity, selectivity and time on stream stability. In the present contribution in situ ATR-IR and ex situ spectroscopic measurements (XPS, UV–vis DRS) have been performed in order to describe the influence of three different oxygenated organic molecules (acetate, citrate, and nitrilotriacetate) on cobalt sorption on a conventional γ-alumina support. Variation of the preparation conditions has been done by changing the pH of adsorption, and the nature of additives (organic molecules) as well as their concentrations. Different sorption mechanisms and cobalt speciation have been evidenced during in situ ATR-IR measurement through comparison with reference spectra of pure Co and organic additives solutions. More specifically, citrate and nitriloacetate additives leads to the formation of ternary surface complexes (alumina–ligand–cobalt) preventing Co precipitation at high pH and leading to a higher Co dispersion at the impregnation step than for the ligand-free system. However, acetate does not sorb on alumina and cannot prevent Co precipitation. However, addition of acetate in the impregnation solution leads to an increase in the amount of cobalt adsorbed at high pH but induces a strong decrease of cobalt sorption at lower pH.
中文翻译:

原位钴形态上的γ-Al 2 ö 3在羧酸根配体的存在下,在负载型催化剂的制备
分子尺度对多孔氧化物载体上金属浸渍的理解是开发优化(且具有成本效益)催化材料的关键一步,该催化材料具有增强的活性,选择性和对流的稳定性的时间。在目前的贡献中,已经进行了原位ATR-IR和异位光谱测量(XPS,UV-vis DRS),以描述三种不同的氧化有机分子(乙酸盐,柠檬酸盐和次氮基三乙酸盐)对钴吸附钴的影响。常规的γ-氧化铝载体。通过改变吸附的pH值,添加剂(有机分子)的性质及其浓度来改变制备条件。通过与纯Co和有机添加剂溶液的参考光谱进行比较,在原位ATR-IR测量过程中已证明了不同的吸附机理和钴形态。更具体地说,柠檬酸和次氮基乙酸盐添加剂会导致三元表面复合物(氧化铝-配体-钴)的形成,从而阻止了高pH条件下Co的沉淀,并导致浸渍步骤中的Co分散度高于无配体体系。但是,乙酸盐不会吸附在氧化铝上,也无法防止Co沉淀。然而,在浸渍溶液中添加乙酸盐导致在高pH下吸附的钴量增加,但是在较低pH下引起钴吸附的强烈下降。柠檬酸盐和次氮基乙酸盐添加剂会导致三元表面复合物(氧化铝-配体-钴)的形成,从而阻止了Co在高pH条件下的沉淀,并导致浸渍步骤中的Co分散度高于无配体体系。但是,乙酸盐不会吸附在氧化铝上,也无法防止Co沉淀。然而,在浸渍溶液中添加乙酸盐导致在高pH下吸附的钴量增加,但是在较低pH下引起钴吸附的强烈下降。柠檬酸盐和次氮基乙酸盐添加剂会导致三元表面复合物(氧化铝-配体-钴)的形成,从而阻止了Co在高pH条件下的沉淀,并导致浸渍步骤中的Co分散度高于无配体体系。但是,乙酸盐不会吸附在氧化铝上,也无法防止Co沉淀。然而,在浸渍溶液中添加乙酸盐导致在高pH下吸附的钴量增加,但是在较低pH下引起钴吸附的强烈下降。
更新日期:2017-09-23
中文翻译:

原位钴形态上的γ-Al 2 ö 3在羧酸根配体的存在下,在负载型催化剂的制备
分子尺度对多孔氧化物载体上金属浸渍的理解是开发优化(且具有成本效益)催化材料的关键一步,该催化材料具有增强的活性,选择性和对流的稳定性的时间。在目前的贡献中,已经进行了原位ATR-IR和异位光谱测量(XPS,UV-vis DRS),以描述三种不同的氧化有机分子(乙酸盐,柠檬酸盐和次氮基三乙酸盐)对钴吸附钴的影响。常规的γ-氧化铝载体。通过改变吸附的pH值,添加剂(有机分子)的性质及其浓度来改变制备条件。通过与纯Co和有机添加剂溶液的参考光谱进行比较,在原位ATR-IR测量过程中已证明了不同的吸附机理和钴形态。更具体地说,柠檬酸和次氮基乙酸盐添加剂会导致三元表面复合物(氧化铝-配体-钴)的形成,从而阻止了高pH条件下Co的沉淀,并导致浸渍步骤中的Co分散度高于无配体体系。但是,乙酸盐不会吸附在氧化铝上,也无法防止Co沉淀。然而,在浸渍溶液中添加乙酸盐导致在高pH下吸附的钴量增加,但是在较低pH下引起钴吸附的强烈下降。柠檬酸盐和次氮基乙酸盐添加剂会导致三元表面复合物(氧化铝-配体-钴)的形成,从而阻止了Co在高pH条件下的沉淀,并导致浸渍步骤中的Co分散度高于无配体体系。但是,乙酸盐不会吸附在氧化铝上,也无法防止Co沉淀。然而,在浸渍溶液中添加乙酸盐导致在高pH下吸附的钴量增加,但是在较低pH下引起钴吸附的强烈下降。柠檬酸盐和次氮基乙酸盐添加剂会导致三元表面复合物(氧化铝-配体-钴)的形成,从而阻止了Co在高pH条件下的沉淀,并导致浸渍步骤中的Co分散度高于无配体体系。但是,乙酸盐不会吸附在氧化铝上,也无法防止Co沉淀。然而,在浸渍溶液中添加乙酸盐导致在高pH下吸附的钴量增加,但是在较低pH下引起钴吸附的强烈下降。


























 京公网安备 11010802027423号
京公网安备 11010802027423号