当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methane C–H Activation via 3d Metal Methoxide Complexes with Potentially Redox-Noninnocent Pincer Ligands: A Density Functional Theory Study
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01736 Ahmad Najafian 1 , Thomas R. Cundari 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01736 Ahmad Najafian 1 , Thomas R. Cundari 1
Affiliation
|
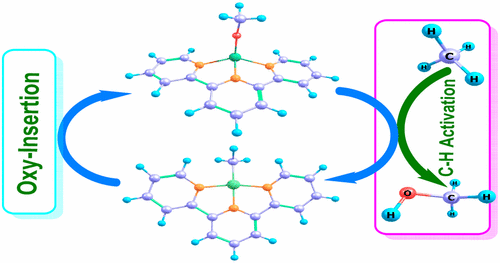
This paper reports a density functional theory study of 3d transition-metal methoxide complexes with potentially redox-noninnocent pincer supporting ligands for methane C–H bond activation to form methanol (LnM-OMe + CH4 → LnM–Me + CH3OH). The three types of tridentate pincer ligands [terpyridine (NNN), bis(2-pyridyl)phenyl-C,N,N′ (NCN), and 2,6-bis(2-phenyl)pyridine-N,C,C′ (CNC)] and different first-row transition metals (M = Ti, V, Cr, Mn, Fe, Co, Ni, and Cu) are used to elucidate the reaction mechanism as well as the effect of the metal identity on the thermodynamics and kinetics of a methane activation reaction. Spin-density analysis indicates that some of these systems, the NNN and NCN ligands, have redox-noninnocent character. A four-centered, kite-shaped transition state, σ-bond metathesis, or oxidative hydrogen migration has been found for methane activation for the complexes studied. Calculations suggest that the d electron count is a more significant factor than the metal formal charge in controlling the thermodynamics and kinetics of C–H activation and late 3d metal methoxides, with high d counts preferred. Notably, early-to-middle metals tend toward oxidative hydrogen migration and late metals undergo a pathway that is more akin to σ-bond metathesis, suggesting that metal methoxide complexes that favor σ-bond metathesis pathways for methane activation will yield lower barriers for C–H activation.
中文翻译:

通过具有潜在的氧化还原-非无损钳形配体的3d金属甲醇络合物活化甲烷C-H:密度泛函理论研究
本文报道了密度泛函理论研究的3d过渡金属甲醇盐配合物,该配合物具有潜在的氧化还原-非无损钳位配体,可通过甲烷配体CH键活化形成甲醇(L n M-OMe + CH 4 →L n M-Me + CH 3 OH)。三种类型的三齿钳状配体[吡啶(NNN),双(2-吡啶基)苯基-C,N,N '(NCN)和2,6-双(2-苯基)吡啶-N,C,C'(CNC)]和不同的第一行过渡金属(M = Ti,V,Cr,Mn,Fe,Co,Ni和Cu)用于阐明反应机理以及金属身份对金属的影响。甲烷活化反应的热力学和动力学。自旋密度分析表明,其中一些系统,即NNN和NCN配体,具有氧化还原非清纯的特性。已经发现了四中心,风筝状的过渡态,σ键易位或氧化氢迁移,可用于所研究的配合物的甲烷活化。计算表明,在控制C–H活化和后期3d金属甲醇盐的热力学和动力学方面,d电子计数比金属形式电荷更重要,其中较高的d计数是可取的。尤其,
更新日期:2017-09-22
中文翻译:

通过具有潜在的氧化还原-非无损钳形配体的3d金属甲醇络合物活化甲烷C-H:密度泛函理论研究
本文报道了密度泛函理论研究的3d过渡金属甲醇盐配合物,该配合物具有潜在的氧化还原-非无损钳位配体,可通过甲烷配体CH键活化形成甲醇(L n M-OMe + CH 4 →L n M-Me + CH 3 OH)。三种类型的三齿钳状配体[吡啶(NNN),双(2-吡啶基)苯基-C,N,N '(NCN)和2,6-双(2-苯基)吡啶-N,C,C'(CNC)]和不同的第一行过渡金属(M = Ti,V,Cr,Mn,Fe,Co,Ni和Cu)用于阐明反应机理以及金属身份对金属的影响。甲烷活化反应的热力学和动力学。自旋密度分析表明,其中一些系统,即NNN和NCN配体,具有氧化还原非清纯的特性。已经发现了四中心,风筝状的过渡态,σ键易位或氧化氢迁移,可用于所研究的配合物的甲烷活化。计算表明,在控制C–H活化和后期3d金属甲醇盐的热力学和动力学方面,d电子计数比金属形式电荷更重要,其中较高的d计数是可取的。尤其,



























 京公网安备 11010802027423号
京公网安备 11010802027423号