当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Organocatalytic One-Pot, Two-Step Sequential Process to Synthesize Chiral Acetal-Containing Polycyclic Derivatives from Cyclic Hemiacetals and Enones
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.joc.7b01915 Chao Liu 1 , Yan-Kai Liu 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.joc.7b01915 Chao Liu 1 , Yan-Kai Liu 1, 2
Affiliation
|
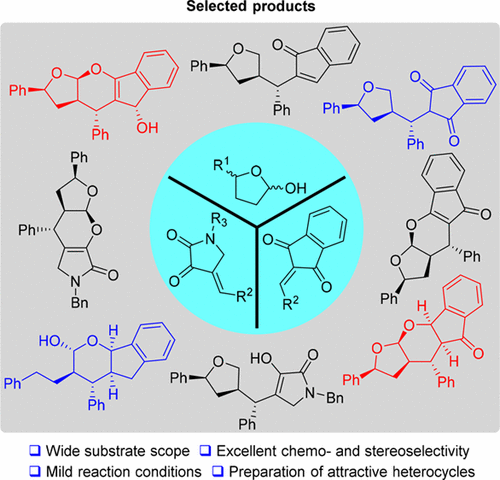
We have developed an efficient one-pot, two-step sequential process to synthesize biologically and synthetically important chiral acetal-containing polycyclic derivatives. This novel protocol had been proved to proceed via Michael-lactolization-oxocarbenium ion ring-closing sequence, which was initiated by a key reactive enamine intermediate and interrupted the previously established reaction pathway of two different enones used in this work, and generated the corresponding cycloadducts with excellent stereoselectivity bearing up to seven continuous stereocenters. Both chiral and racemic starting cyclic hemiacetals worked well in this strategy. The synthetic applications of the obtained polycyclic products have also been demonstrated.
中文翻译:

不对称有机催化一锅两步顺序过程从环状半缩醛和烯酮合成手性含缩醛的多环衍生物
我们已经开发了一种有效的一锅,两步顺序方法,可以合成生物学上和合成上重要的手性含缩醛的多环衍生物。事实证明,该新方案可通过迈克尔-乳化-氧碳鎓离子闭环序列进行,该过程由关键的反应性烯胺中间体引发,中断了先前在该工作中使用的两种不同烯酮的反应路径,并生成了相应的环加合物具有出色的立体选择性,最多可容纳七个连续的立体中心。手性和外消旋起始环状半缩醛在该策略中均能很好地发挥作用。还证明了所得多环产物的合成应用。
更新日期:2017-09-22
中文翻译:

不对称有机催化一锅两步顺序过程从环状半缩醛和烯酮合成手性含缩醛的多环衍生物
我们已经开发了一种有效的一锅,两步顺序方法,可以合成生物学上和合成上重要的手性含缩醛的多环衍生物。事实证明,该新方案可通过迈克尔-乳化-氧碳鎓离子闭环序列进行,该过程由关键的反应性烯胺中间体引发,中断了先前在该工作中使用的两种不同烯酮的反应路径,并生成了相应的环加合物具有出色的立体选择性,最多可容纳七个连续的立体中心。手性和外消旋起始环状半缩醛在该策略中均能很好地发挥作用。还证明了所得多环产物的合成应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号