当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
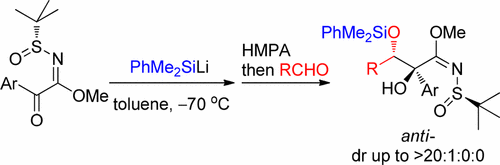
Reaction of Silyllithium, α-Keto N-tert-Butanesulfinyl Imidates and Aldehydes for Asymmetric Synthesis of α-Substituted β-(Silyloxy)-α-hydroxy Acid Derivatives
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.joc.7b02214 Wei Huang 1, 2 , Hui Liu 1 , Yan-Jun Xu 1 , Chong-Dao Lu 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.joc.7b02214 Wei Huang 1, 2 , Hui Liu 1 , Yan-Jun Xu 1 , Chong-Dao Lu 1
Affiliation
|
A single-flask reaction of silyllithium, α-keto N-tert-butanesulfinyl imidates and aldehydes has been developed for the diastereoselective synthesis of α,β-dihydroxy acid derivatives. In this reaction, the nucleophilic addition of silyllithium to chiral α-keto imidates followed by silyl migration forms chiral aza-enolates, which react diastereoselectively with aldehydes. Subsequent [1,4]-O → O silyl migration affords α-substituted β-(silyoxy)-α-hydroxy imidates.
中文翻译:

的Silyllithium,α酮反应ñ -叔-Butanesulfinyl亚氨酸酯和醛为α位取代β-(甲硅烷氧基)-α-羟基酸衍生物的不对称合成
的silyllithium,α酮单烧瓶反应ñ -叔-butanesulfinyl亚氨酸酯和醛已为α,β二羟基酸衍生物的非对映选择性合成的发展。在该反应中,甲硅烷基锂亲核加成到手性α-酮酰亚胺上,然后甲硅烷基迁移形成手性氮杂-烯醇盐,它们与醛非对映选择性地反应。随后的[1,4] -O→O甲硅烷基迁移得到α-取代的β-(甲硅烷氧基)-α-羟基酰亚胺。
更新日期:2017-09-22
中文翻译:

的Silyllithium,α酮反应ñ -叔-Butanesulfinyl亚氨酸酯和醛为α位取代β-(甲硅烷氧基)-α-羟基酸衍生物的不对称合成
的silyllithium,α酮单烧瓶反应ñ -叔-butanesulfinyl亚氨酸酯和醛已为α,β二羟基酸衍生物的非对映选择性合成的发展。在该反应中,甲硅烷基锂亲核加成到手性α-酮酰亚胺上,然后甲硅烷基迁移形成手性氮杂-烯醇盐,它们与醛非对映选择性地反应。随后的[1,4] -O→O甲硅烷基迁移得到α-取代的β-(甲硅烷氧基)-α-羟基酰亚胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号