当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Visible light-induced C–H sulfenylation using sulfinic acids
Green Chemistry ( IF 9.8 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1039/c7gc01891f Pengfei Sun 1, 2, 3, 4, 5 , Daoshan Yang 1, 2, 3, 4, 5 , Wei Wei 1, 2, 3, 4, 5 , Min Jiang 6, 7, 8, 9, 10 , Zuli Wang 5, 11, 12, 13 , Liyan Zhang 1, 2, 3, 4, 5 , Hui Zhang 1, 2, 3, 4, 5 , Zhenzhen Zhang 1, 2, 3, 4, 5 , Yu Wang 1, 2, 3, 4, 5 , Hua Wang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1039/c7gc01891f Pengfei Sun 1, 2, 3, 4, 5 , Daoshan Yang 1, 2, 3, 4, 5 , Wei Wei 1, 2, 3, 4, 5 , Min Jiang 6, 7, 8, 9, 10 , Zuli Wang 5, 11, 12, 13 , Liyan Zhang 1, 2, 3, 4, 5 , Hui Zhang 1, 2, 3, 4, 5 , Zhenzhen Zhang 1, 2, 3, 4, 5 , Yu Wang 1, 2, 3, 4, 5 , Hua Wang 1, 2, 3, 4, 5
Affiliation
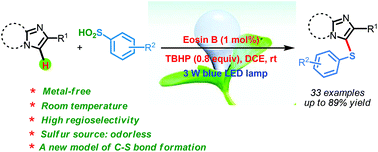
|
The visible light and Eosin B-catalyzed direct sulfenylation of sp2 C–H bonds with sulfinic acids through a photoredox process has been demonstrated at room temperature under transition metal-free conditions for the first time. Diverse heteroaryl sulfides were obtained in moderate to good yields. This is the first example of the sulfenylation of sp2 C–H bonds using arylsulfinic acids as odorless sulfur reagents under visible light-induced conditions. More interestingly, the reductive products could be obtained under visible light-induced oxidative conditions. This protocol demonstrates a new model for C–S bond formation, which serves as a novel approach toward the synthesis of heteroaryl sulfides.
中文翻译:

亚磺酸使可见光诱导的CH H亚磺酰化
首次在室温下,无过渡金属的条件下,证明了可见光和曙红B催化的亚砜酸通过光氧化还原过程使sp 2 C–H键与亚磺酸发生直接亚磺基化反应。以中等至良好的产率获得了多种杂芳基硫化物。这是在可见光诱导的条件下,使用芳基亚磺酸作为无味硫试剂对sp 2 C–H键进行亚磺酰基化的第一个例子。更有趣的是,可以在可见光诱导的氧化条件下获得还原产物。该协议展示了一个用于C–S键形成的新模型,该模型为合成杂芳基硫醚提供了一种新颖的方法。
更新日期:2017-09-22
中文翻译:

亚磺酸使可见光诱导的CH H亚磺酰化
首次在室温下,无过渡金属的条件下,证明了可见光和曙红B催化的亚砜酸通过光氧化还原过程使sp 2 C–H键与亚磺酸发生直接亚磺基化反应。以中等至良好的产率获得了多种杂芳基硫化物。这是在可见光诱导的条件下,使用芳基亚磺酸作为无味硫试剂对sp 2 C–H键进行亚磺酰基化的第一个例子。更有趣的是,可以在可见光诱导的氧化条件下获得还原产物。该协议展示了一个用于C–S键形成的新模型,该模型为合成杂芳基硫醚提供了一种新颖的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号