当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Knudsen cell studies of the uptake of gaseous ammonia and amines onto C3–C7 solid dicarboxylic acids
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1039/c7cp05252a Michelle C. Fairhurst 1, 2, 3 , Michael J. Ezell 1, 2, 3 , Barbara J. Finlayson-Pitts 1, 2, 3
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1039/c7cp05252a Michelle C. Fairhurst 1, 2, 3 , Michael J. Ezell 1, 2, 3 , Barbara J. Finlayson-Pitts 1, 2, 3
Affiliation
|
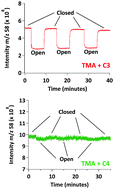
While atmospheric particles affect health, visibility and climate, the details governing their formation and growth are poorly understood on a molecular level. A simple model system for understanding the interactions between the gas and particle phases is the reaction of bases with acids, both of which are common constituents of atmospheric particles. In the present study, uptake coefficients for the reactions of gas phase ammonia, methylamine, ethylamine, dimethylamine and trimethylamine with a series of solid dicarboxylic acids (diacids) were measured at 296 ± 1 K using a Knudsen cell interfaced to a quadrupole mass spectrometer. The uptake coefficients (γ) for a given amine follow an odd–even trend in carbon number of the diacid, and are larger for the odd carbon diacids. Values range from γ = 0.4 for ethylamine on malonic acid (C3) to less than ∼10−6 for ammonia and all amines on adipic (C6) and pimelic (C7) acids. Basicity or structure of the amines/ammonia alone do not explain the effect of the base on uptake. The crystal structures of the diacids also play a key role, which is especially evident for malonic acid (C3). Evaporation of aqueous mixtures of amines/ammonia with odd carbon diacids show the formation of ionic liquids (ILs) or in some cases, metastable ILs that revert back to a stable solid salt upon complete evaporation of water. The trends with amine and diacid structure provide insight into the mechanisms of uptake and molecular interactions that control it, including the formation of ionic liquid layers in some cases. The diversity in the kinetics and mechanisms involved in this relatively simple model system illustrate the challenges in accurately representing such processes in atmospheric models.
中文翻译:

Knudsen细胞研究气态氨和胺被C3-C7固体二羧酸吸收的过程
尽管大气颗粒会影响健康,能见度和气候,但在分子水平上对控制其形成和生长的细节知之甚少。理解气相和粒子相之间相互作用的一个简单模型系统是碱与酸的反应,两者都是大气粒子的常见成分。在本研究中,使用与四极杆质谱仪连接的Knudsen池在296±1 K下测量了气相氨,甲胺,乙胺,二甲胺和三甲胺与一系列固体二羧酸(二酸)的反应的吸收系数。给定胺的吸收系数(γ)遵循二酸碳数的奇偶趋势,而对于奇数碳二酸则更大。值范围从γ丙二酸(C3)上的乙胺= 0.4至小于〜10 -6用于己二酸(C6)和庚二酸(C7)上的氨和所有胺。单独的胺/氨的碱性或结构不能解释碱对摄取的影响。二元酸的晶体结构也起关键作用,这对于丙二酸(C3)尤其明显。胺/氨与奇数碳二酸的水性混合物的蒸发表明形成了离子液体(ILs),或者在某些情况下,亚稳的ILs在水完全蒸发后又恢复为稳定的固体盐。胺和二酸结构的变化趋势提供了控制其吸收和分子相互作用的机理的见解,在某些情况下还包括形成离子液体层。
更新日期:2017-09-22
中文翻译:

Knudsen细胞研究气态氨和胺被C3-C7固体二羧酸吸收的过程
尽管大气颗粒会影响健康,能见度和气候,但在分子水平上对控制其形成和生长的细节知之甚少。理解气相和粒子相之间相互作用的一个简单模型系统是碱与酸的反应,两者都是大气粒子的常见成分。在本研究中,使用与四极杆质谱仪连接的Knudsen池在296±1 K下测量了气相氨,甲胺,乙胺,二甲胺和三甲胺与一系列固体二羧酸(二酸)的反应的吸收系数。给定胺的吸收系数(γ)遵循二酸碳数的奇偶趋势,而对于奇数碳二酸则更大。值范围从γ丙二酸(C3)上的乙胺= 0.4至小于〜10 -6用于己二酸(C6)和庚二酸(C7)上的氨和所有胺。单独的胺/氨的碱性或结构不能解释碱对摄取的影响。二元酸的晶体结构也起关键作用,这对于丙二酸(C3)尤其明显。胺/氨与奇数碳二酸的水性混合物的蒸发表明形成了离子液体(ILs),或者在某些情况下,亚稳的ILs在水完全蒸发后又恢复为稳定的固体盐。胺和二酸结构的变化趋势提供了控制其吸收和分子相互作用的机理的见解,在某些情况下还包括形成离子液体层。



























 京公网安备 11010802027423号
京公网安备 11010802027423号