当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Stereoablative Aryloxylation of 3-Bromooxindoles with Bifunctional Catalyst
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-09-21 03:30:49 , DOI: 10.1002/adsc.201700693 Amol P. Jadhav 1 , Aarti Manchanda 1 , Manish K. Jaiswal 1 , Ravi P. Singh 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-09-21 03:30:49 , DOI: 10.1002/adsc.201700693 Amol P. Jadhav 1 , Aarti Manchanda 1 , Manish K. Jaiswal 1 , Ravi P. Singh 1
Affiliation
|
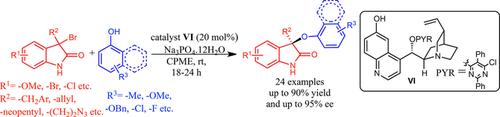
In this communication, the development of a highly enantioselective stereoablative protocol for the chemoselective aryloxylation of 3-bromooxindoles with aryl alcohols have been realized. Promoted by easily available cinchona alkaloid derivatives (C6′−OH) under air- and moisture-tolerant conditions, aryloxylation of a wide range of 3-bromooxindoles bearing a 3′-alkyl substituent proceeded in high yields and excellent enantioselectivities (up to 95% ee). The newly developed strategy expediently provided the 3-phenoxy analogue of the core of alkaloid CPC-1.
中文翻译:

双官能团催化剂催化3-溴恶吲哚的不对称立体合成芳氧基化
在这种交流中,已经实现了用于3-溴氧吲哚与芳基醇的化学选择性芳氧基化的高度对映选择性立体消融方案的开发。由易于获得的金鸡纳生物碱衍生物(C6'-OH)在耐空气和潮气的条件下促进的,带有3'-烷基取代基的各种3-溴代吲哚的芳氧基化反应具有很高的收率和出色的对映选择性(高达95%) ee)。新开发的策略方便地提供了生物碱CPC-1核心的3-苯氧基类似物。
更新日期:2017-09-21
中文翻译:

双官能团催化剂催化3-溴恶吲哚的不对称立体合成芳氧基化
在这种交流中,已经实现了用于3-溴氧吲哚与芳基醇的化学选择性芳氧基化的高度对映选择性立体消融方案的开发。由易于获得的金鸡纳生物碱衍生物(C6'-OH)在耐空气和潮气的条件下促进的,带有3'-烷基取代基的各种3-溴代吲哚的芳氧基化反应具有很高的收率和出色的对映选择性(高达95%) ee)。新开发的策略方便地提供了生物碱CPC-1核心的3-苯氧基类似物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号