当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral Ramachandran Plots I: Glycine
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00525 Yael Baruch-Shpigler 1 , Huan Wang 1, 2 , Inbal Tuvi-Arad 2 , David Avnir 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00525 Yael Baruch-Shpigler 1 , Huan Wang 1, 2 , Inbal Tuvi-Arad 2 , David Avnir 1
Affiliation
|
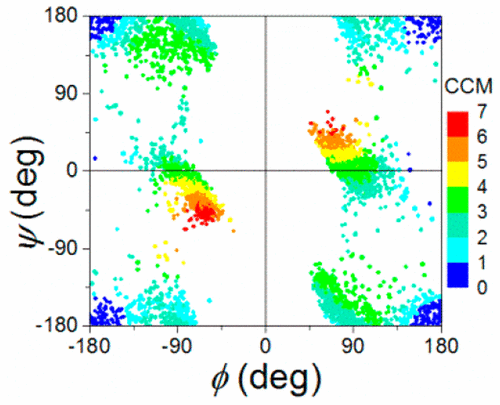
Ramachandran plots (RPs) map the wealth of conformations of the polypeptide backbone and are widely used to characterize protein structures. A limitation of the RPs is that they are based solely on two dihedral angles for each amino acid residue and provide therefore only a partial picture of the conformational richness of the protein. Here we extend the structural RP analysis of proteins from a two-dimensional (2D) map to a three-dimensional map by adding the quantitative degree of chirality—the continuous chirality measure (CCM)—of the amino acid residue at each point in the RP. This measure encompasses all bond angles and bond lengths of an amino acid residue. We focus in this report on glycine (Gly) because, due to its flexibility, it occupies a large portion of the 2D map, thus allowing a detailed study of the chirality measure, and in order to evaluate the justification of classically labeling Gly as the only achiral amino acid. We have analyzed in detail 4366 Gly residues extracted from high resolution crystallographic data of 160 proteins. This analysis reveals not only that Gly is practically always conformationally chiral, but that upon comparing with the backbone of all amino acids, the quantitative chirality values of Gly are of similar magnitudes to those of the (chiral) amino acids. Structural trends and energetic considerations are discussed in detail. Generally we show that adding chirality to Ramachandran plots creates far more informative plots that highlight the sensitivity of the protein structure to minor conformational changes.
中文翻译:

手性Ramachandran图I:甘氨酸
Ramachandran图(RPs)绘制了多肽主链的丰富构象,被广泛用于表征蛋白质结构。RP的局限性在于它们仅基于每个氨基酸残基的两个二面角,因此仅提供了蛋白质构象丰富度的部分图片。在这里,我们通过添加蛋白质在每个点的氨基酸残基的定量手性(连续手性度量(CCM)),将蛋白质的结构RP分析从二维(2D)图扩展到三维图。 RP。该量度涵盖氨基酸残基的所有键角和键长。我们在本报告中重点介绍甘氨酸(Gly),因为由于其灵活性,它占据了2D地图的很大一部分,因此可以详细研究手性方法,为了评估经典地将Gly标记为唯一的非手性氨基酸的理由。我们已经详细分析了从160种蛋白质的高分辨率晶体学数据中提取的4366个Gly残基。该分析不仅揭示了Gly实际上总是构象手性的,而且与所有氨基酸的主链相比,Gly的定量手性值与(手性)氨基酸的数量手性值具有相似的大小。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。我们已经详细分析了从160种蛋白质的高分辨率晶体学数据中提取的4366个Gly残基。该分析不仅揭示了Gly实际上总是构象手性的,而且与所有氨基酸的主链相比,Gly的定量手性值与(手性)氨基酸的数量手性值具有相似的大小。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。我们已经详细分析了从160种蛋白质的高分辨率晶体学数据中提取的4366个Gly残基。该分析不仅揭示了Gly实际上总是构象手性的,而且与所有氨基酸的主链相比,Gly的定量手性值与(手性)氨基酸的数量手性值具有相似的大小。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。Gly的定量手性值具有与(手性)氨基酸相似的数量级。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,向Ramachandran图中添加手性会创建更多信息的图,这些图突出了蛋白质结构对微小构象变化的敏感性。Gly的定量手性值具有与(手性)氨基酸相似的数量级。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。
更新日期:2017-09-21
中文翻译:

手性Ramachandran图I:甘氨酸
Ramachandran图(RPs)绘制了多肽主链的丰富构象,被广泛用于表征蛋白质结构。RP的局限性在于它们仅基于每个氨基酸残基的两个二面角,因此仅提供了蛋白质构象丰富度的部分图片。在这里,我们通过添加蛋白质在每个点的氨基酸残基的定量手性(连续手性度量(CCM)),将蛋白质的结构RP分析从二维(2D)图扩展到三维图。 RP。该量度涵盖氨基酸残基的所有键角和键长。我们在本报告中重点介绍甘氨酸(Gly),因为由于其灵活性,它占据了2D地图的很大一部分,因此可以详细研究手性方法,为了评估经典地将Gly标记为唯一的非手性氨基酸的理由。我们已经详细分析了从160种蛋白质的高分辨率晶体学数据中提取的4366个Gly残基。该分析不仅揭示了Gly实际上总是构象手性的,而且与所有氨基酸的主链相比,Gly的定量手性值与(手性)氨基酸的数量手性值具有相似的大小。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。我们已经详细分析了从160种蛋白质的高分辨率晶体学数据中提取的4366个Gly残基。该分析不仅揭示了Gly实际上总是构象手性的,而且与所有氨基酸的主链相比,Gly的定量手性值与(手性)氨基酸的数量手性值具有相似的大小。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。我们已经详细分析了从160种蛋白质的高分辨率晶体学数据中提取的4366个Gly残基。该分析不仅揭示了Gly实际上总是构象手性的,而且与所有氨基酸的主链相比,Gly的定量手性值与(手性)氨基酸的数量手性值具有相似的大小。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。Gly的定量手性值具有与(手性)氨基酸相似的数量级。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,向Ramachandran图中添加手性会创建更多信息的图,这些图突出了蛋白质结构对微小构象变化的敏感性。Gly的定量手性值具有与(手性)氨基酸相似的数量级。详细讨论了结构趋势和充满活力的注意事项。通常,我们表明,将手性添加到Ramachandran图中会创建更多的信息图,突出显示了蛋白质结构对微小构象变化的敏感性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号