当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Uric Acid as an Electrochemically Active Compound for Sodium-Ion Batteries: Stepwise Na+-Storage Mechanisms of π-Conjugation and Stabilized Carbon Anion
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acsami.7b10165 Chao Ma 1 , Xiaolin Zhao 2 , Michelle M. Harris 1 , Jianjun Liu 2 , Kai-Xue Wang 1 , Jie-Sheng Chen 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acsami.7b10165 Chao Ma 1 , Xiaolin Zhao 2 , Michelle M. Harris 1 , Jianjun Liu 2 , Kai-Xue Wang 1 , Jie-Sheng Chen 1
Affiliation
|
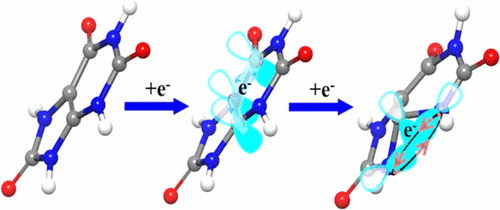
Developing efficient sodium-ion-storage mechanisms to increase the energy capacity in organic electrodes is a critical issue even after this period of prolonged effort. Uric acid (UA), a simple organic compound with three carbonyl groups is demonstrated to be electrochemically active in the insertion/extraction of Na ions. Theoretical calculations and experimental characterizations reveal that the sodium-ion storage by UA is a result of the stepwise mechanisms of p−π conjugation and the carbon anion. Aside from C═O, the functional group C═C(NH—)2 also provides an efficient Na-storage activated site in which the lone-pair electrons is stabilized through the planar-to-tetrahedral structural transition and low-energy orbital hybridization of N atoms. For further improvement of the electrochemical performance, a uric acid and carbon nanotube (UA@CNT) composite is prepared via a vacuum solution impregnation method. When employed as an anode material for sodium-ion batteries, the UA@CNT composite exhibits high specific capacity, excellent rate capability, and long cycling life even at high current densities. A reversible capacity of over 163 mA h g–1 is maintained even after 150 cycles at a current density of 200 mA g–1. The present study paves a way to develop reversible high-capacity organic electrode materials for sodium-ion batteries by a carbon-anion stabilization mechanism.
中文翻译:

尿酸作为钠离子电池的电化学活性化合物:π共轭和稳定碳阴离子的逐步Na +储存机理
即使经过长时间的努力,开发有效的钠离子存储机制来增加有机电极的能量容量也是一个关键问题。尿酸(UA)是具有三个羰基的简单有机化合物,在Na离子的插入/萃取中具有电化学活性。理论计算和实验表征表明,UA的钠离子存储是p-π共轭和碳阴离子逐步机理的结果。除C═O外,官能团C═C(NH-)2还提供了一个有效的钠存储活化位点,其中孤对电子通过平面到四面体的结构转变和N原子的低能轨道杂交而得以稳定。为了进一步提高电化学性能,通过真空溶液浸渍法制备了尿酸和碳纳米管(UA @ CNT)复合材料。UA @ CNT复合材料用作钠离子电池的负极材料时,即使在高电流密度下也具有高比容量,优异的倍率性能和长循环寿命。即使在200 mA g –1的电流密度下经过150次循环,仍可保持超过163 mA hg –1的可逆容量。。本研究为通过碳阴离子稳定机制开发用于钠离子电池的可逆高容量有机电极材料铺平了道路。
更新日期:2017-09-21
中文翻译:

尿酸作为钠离子电池的电化学活性化合物:π共轭和稳定碳阴离子的逐步Na +储存机理
即使经过长时间的努力,开发有效的钠离子存储机制来增加有机电极的能量容量也是一个关键问题。尿酸(UA)是具有三个羰基的简单有机化合物,在Na离子的插入/萃取中具有电化学活性。理论计算和实验表征表明,UA的钠离子存储是p-π共轭和碳阴离子逐步机理的结果。除C═O外,官能团C═C(NH-)2还提供了一个有效的钠存储活化位点,其中孤对电子通过平面到四面体的结构转变和N原子的低能轨道杂交而得以稳定。为了进一步提高电化学性能,通过真空溶液浸渍法制备了尿酸和碳纳米管(UA @ CNT)复合材料。UA @ CNT复合材料用作钠离子电池的负极材料时,即使在高电流密度下也具有高比容量,优异的倍率性能和长循环寿命。即使在200 mA g –1的电流密度下经过150次循环,仍可保持超过163 mA hg –1的可逆容量。。本研究为通过碳阴离子稳定机制开发用于钠离子电池的可逆高容量有机电极材料铺平了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号