当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Proton-Coupled Conformational Allostery Modulates the Inhibitor Selectivity for β-Secretase
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.jpclett.7b02309 Robert C. Harris 1 , Cheng-Chieh Tsai 1 , Christopher R. Ellis 1 , Jana Shen 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.jpclett.7b02309 Robert C. Harris 1 , Cheng-Chieh Tsai 1 , Christopher R. Ellis 1 , Jana Shen 1
Affiliation
|
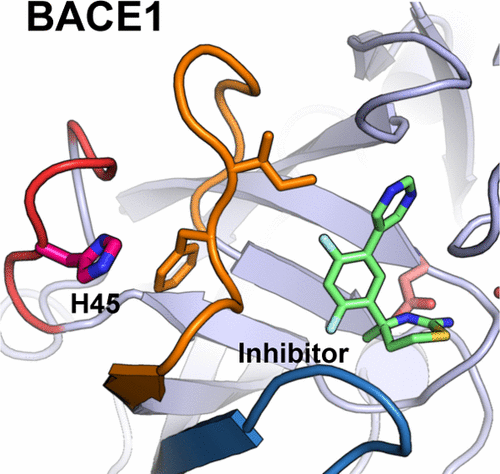
Many important pharmaceutical targets, such as aspartyl proteases and kinases, exhibit pH-dependent dynamics, functions and inhibition. Accurate prediction of their binding free energies is challenging because current computational techniques neglect the effects of pH. Here we combine free energy perturbation calculations with continuous constant pH molecular dynamics to explore the selectivity of a small-molecule inhibitor for β-secretase (BACE1), an important drug target for Alzheimer’s disease. The calculations predicted identical affinity for BACE1 and the closely related cathepsin D at high pH; however, at pH 4.6 the inhibitor is selective for BACE1 by 1.3 kcal/mol, in excellent agreement with experiment. Surprisingly, the pH-dependent selectivity can be attributed to the protonation of His45, which allosterically modulates a loop–inhibitor interaction. Allosteric regulation induced by proton binding is likely common in biology; considering such allosteric sites could lead to exciting new opportunities in drug design.
中文翻译:

质子偶联的构象构象调节β-分泌酶的抑制剂选择性
许多重要的药物靶标,例如天冬氨酰蛋白酶和激酶,都具有pH依赖性的动力学,功能和抑制作用。由于目前的计算技术忽略了pH的影响,因此准确预测其结合自由能具有挑战性。在这里,我们将自由能摄动计算与连续恒定的pH分子动力学相结合,以探索小分子抑制剂对β-分泌酶(BACE1)的选择性,β-分泌酶是阿尔茨海默氏病的重要药物靶标。该计算预测在高pH下对BACE1和紧密相关的组织蛋白酶D具有相同的亲和力。但是,在pH 4.6时,该抑制剂对BACE1的选择性为1.3 kcal / mol,与实验非常吻合。令人惊讶的是,pH依赖性选择性可归因于His45的质子化,变构地调节环-抑制剂相互作用。质子结合引起的变构调节可能在生物学中很常见。考虑到这样的变构位点可能会为药物设计带来令人兴奋的新机会。
更新日期:2017-09-21
中文翻译:

质子偶联的构象构象调节β-分泌酶的抑制剂选择性
许多重要的药物靶标,例如天冬氨酰蛋白酶和激酶,都具有pH依赖性的动力学,功能和抑制作用。由于目前的计算技术忽略了pH的影响,因此准确预测其结合自由能具有挑战性。在这里,我们将自由能摄动计算与连续恒定的pH分子动力学相结合,以探索小分子抑制剂对β-分泌酶(BACE1)的选择性,β-分泌酶是阿尔茨海默氏病的重要药物靶标。该计算预测在高pH下对BACE1和紧密相关的组织蛋白酶D具有相同的亲和力。但是,在pH 4.6时,该抑制剂对BACE1的选择性为1.3 kcal / mol,与实验非常吻合。令人惊讶的是,pH依赖性选择性可归因于His45的质子化,变构地调节环-抑制剂相互作用。质子结合引起的变构调节可能在生物学中很常见。考虑到这样的变构位点可能会为药物设计带来令人兴奋的新机会。


























 京公网安备 11010802027423号
京公网安备 11010802027423号