当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning the synthesis of fully conjugated block copolymers to minimize architectural heterogeneity
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7ta06758e Youngmin Lee 1, 2, 3, 4 , Melissa P. Aplan 1, 2, 3, 4 , Zach D. Seibers 4, 5, 6, 7 , S. Michael Kilbey 4, 5, 6, 7, 8 , Qing Wang 2, 3, 4, 9 , Enrique D. Gomez 1, 2, 3, 4, 10
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7ta06758e Youngmin Lee 1, 2, 3, 4 , Melissa P. Aplan 1, 2, 3, 4 , Zach D. Seibers 4, 5, 6, 7 , S. Michael Kilbey 4, 5, 6, 7, 8 , Qing Wang 2, 3, 4, 9 , Enrique D. Gomez 1, 2, 3, 4, 10
Affiliation
|
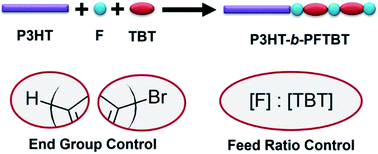
Conjugated block copolymers simultaneously control the mesoscale morphology and interfacial structure of the active layer in organic electronic devices. Fully conjugated block copolymers, where both backbones are conjugated, are commonly synthesized in two steps. First, poly(3-alkylthiophene-2,5-diyl) (P3HT) is synthesized by Kumada catalyst transfer/Grignard metathesis polymerization. The second block, typically a push–pull alternating copolymer, is added on to the P3HT macroreagent in a chain-extension reaction using either a Suzuki or a Stille polycondensation. Consequently, products can be a mixture of homopolymers, diblock copolymers, and multi-block copolymers. We demonstrate the optimum reaction conditions for the two-step synthesis of poly(3-hexylthiophene-2,5-diyl)-block-poly((9,9-bis-(2-octyl)fluorene-2,7-diyl)-alt-(4,7-di(thiophene-2-yl)-2,1,3-benzothiadiazole)-5′,5′′-diyl) (P3HT-b-PFTBT), a block copolymer that can be used as the sole active-layer material in organic photovoltaic devices. In the first reaction, preventing excess Grignard reagent to avert excess in the stoichiometry between Grignard reagent and monomer ensures end-group control of the P3HT macroreagent. In the second reaction, asymmetric monomer feed ratios with excess fluorene promotes coupling of PFTBT to P3HT. Using P3HT-b-PFTBT as an example, we demonstrate the synthetic parameters that are important to produce diblock copolymers with minimal impurities. This, in turn, promotes microphase separation in block copolymer films and leads to enhanced power conversion efficiencies in block copolymer solar cell devices.
中文翻译:

调整全共轭嵌段共聚物的合成,以最大程度地减少结构异质性
共轭嵌段共聚物可同时控制有机电子器件中活性层的介观形貌和界面结构。通常将两个骨架共轭的全共轭嵌段共聚物合成。首先,通过Kumada催化剂转移/ Grignard易位聚合合成聚(3-烷基噻吩-2,5-二基)(P3HT)。第二个嵌段(通常为推挽交替共聚物)通过Suzuki或Stille缩聚反应在链延长反应中添加到P3HT大分子试剂上。因此,产物可以是均聚物,二嵌段共聚物和多嵌段共聚物的混合物。我们证明了两步合成聚(3-己基噻吩-2,5-二基)-嵌段的最佳反应条件-聚((9,9-双(2-辛基)芴-2,7-二基) - ALT - (4,7-二(噻吩-2-基)-2,1,3-苯并噻二唑)-5- ',5''-二基)(P3HT- b -PFTBT),一种嵌段共聚物,可以用作有机光伏器件中的唯一活性层材料。在第一个反应中,防止过量的格氏试剂避免格氏试剂和单体之间化学计量的过量,可确保对P3HT大分子试剂的端基控制。在第二反应中,具有过量芴的不对称单体进料比促进了PFTBT与P3HT的偶联。使用P3HT- b以-PFTBT为例,我们证明了合成参数对于生产具有最少杂质的二嵌段共聚物非常重要。反过来,这促进了嵌段共聚物膜中的微相分离,并导致了嵌段共聚物太阳能电池器件中功率转换效率的提高。
更新日期:2017-09-21
中文翻译:

调整全共轭嵌段共聚物的合成,以最大程度地减少结构异质性
共轭嵌段共聚物可同时控制有机电子器件中活性层的介观形貌和界面结构。通常将两个骨架共轭的全共轭嵌段共聚物合成。首先,通过Kumada催化剂转移/ Grignard易位聚合合成聚(3-烷基噻吩-2,5-二基)(P3HT)。第二个嵌段(通常为推挽交替共聚物)通过Suzuki或Stille缩聚反应在链延长反应中添加到P3HT大分子试剂上。因此,产物可以是均聚物,二嵌段共聚物和多嵌段共聚物的混合物。我们证明了两步合成聚(3-己基噻吩-2,5-二基)-嵌段的最佳反应条件-聚((9,9-双(2-辛基)芴-2,7-二基) - ALT - (4,7-二(噻吩-2-基)-2,1,3-苯并噻二唑)-5- ',5''-二基)(P3HT- b -PFTBT),一种嵌段共聚物,可以用作有机光伏器件中的唯一活性层材料。在第一个反应中,防止过量的格氏试剂避免格氏试剂和单体之间化学计量的过量,可确保对P3HT大分子试剂的端基控制。在第二反应中,具有过量芴的不对称单体进料比促进了PFTBT与P3HT的偶联。使用P3HT- b以-PFTBT为例,我们证明了合成参数对于生产具有最少杂质的二嵌段共聚物非常重要。反过来,这促进了嵌段共聚物膜中的微相分离,并导致了嵌段共聚物太阳能电池器件中功率转换效率的提高。


























 京公网安备 11010802027423号
京公网安备 11010802027423号