当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogen evolution reaction activity of nickel phosphide is highly sensitive to electrolyte pH
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7ta06000a Zheng Zhou 1, 2, 3, 4 , Li Wei 1, 2, 3, 4 , Yanqing Wang 5, 6, 7, 8 , H. Enis Karahan 9, 10, 11 , Zibin Chen 1, 3, 4, 12, 13 , Yaojie Lei 1, 2, 3, 4 , Xuncai Chen 1, 2, 3, 4 , Shengli Zhai 1, 2, 3, 4, 9 , Xiaozhou Liao 1, 3, 4, 12, 13 , Yuan Chen 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7ta06000a Zheng Zhou 1, 2, 3, 4 , Li Wei 1, 2, 3, 4 , Yanqing Wang 5, 6, 7, 8 , H. Enis Karahan 9, 10, 11 , Zibin Chen 1, 3, 4, 12, 13 , Yaojie Lei 1, 2, 3, 4 , Xuncai Chen 1, 2, 3, 4 , Shengli Zhai 1, 2, 3, 4, 9 , Xiaozhou Liao 1, 3, 4, 12, 13 , Yuan Chen 1, 2, 3, 4
Affiliation
|
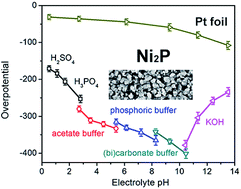
The nickel phosphide (Ni2P) family of materials have become a hot subject in hydrogen evolution reaction (HER) electrocatalyst research. Various studies have reported their high activity, high stability, and high faradaic efficiency. To date, there have been no systematic studies regarding the influence of pH on the HER performance of Ni2P. Here we show that the pH of electrolytes can strongly influence the HER activity of Ni2P electrocatalysts. Tests in 19 electrolytes with pH ranging from 0.52 to 13.53 show that Ni2P is much more active in strongly acidic and basic electrolytes. With the increase of pH, the lower H+ concentration reduces the formation of adsorbed H atoms in the Volmer reaction, resulting in poorer activities. However, the high activity observed in the strongly basic electrolytes is not the intrinsic property of Ni2P. We found that Ni oxides/hydroxides are formed in strongly basic electrolytes under applied potentials, resulting in improved activities. Furthermore, the specific activity based on the electrochemically active surface area of recently reported Ni2P catalysts is not high and requires significant improvements for practical applications.
中文翻译:

磷化镍的析氢反应活性对电解质pH高度敏感
磷化镍(Ni 2 P)系列材料已成为氢析出反应(HER)电催化剂研究的热点。各种研究报告了它们的高活性,高稳定性和高法拉第效率。迄今为止,还没有关于pH对Ni 2 P的HER性能的影响的系统研究。在这里,我们表明电解质的pH值可以强烈影响Ni 2 P电催化剂的HER活性。在19种pH值为0.52至13.53的电解质中进行的测试表明,Ni 2 P在强酸性和碱性电解质中的活性更高。随着pH值的增加,H +降低浓度降低了沃尔默反应中吸附的H原子的形成,从而导致较差的活性。然而,在强碱性电解质中观察到的高活性不是Ni 2 P的固有性质。我们发现,在强电势下,在施加电势下会形成Ni氧化物/氢氧化物,从而提高了活性。此外,基于最近报道的Ni 2 P催化剂的电化学活性表面积的比活性不高,并且对于实际应用需要显着的改进。
更新日期:2017-09-21
中文翻译:

磷化镍的析氢反应活性对电解质pH高度敏感
磷化镍(Ni 2 P)系列材料已成为氢析出反应(HER)电催化剂研究的热点。各种研究报告了它们的高活性,高稳定性和高法拉第效率。迄今为止,还没有关于pH对Ni 2 P的HER性能的影响的系统研究。在这里,我们表明电解质的pH值可以强烈影响Ni 2 P电催化剂的HER活性。在19种pH值为0.52至13.53的电解质中进行的测试表明,Ni 2 P在强酸性和碱性电解质中的活性更高。随着pH值的增加,H +降低浓度降低了沃尔默反应中吸附的H原子的形成,从而导致较差的活性。然而,在强碱性电解质中观察到的高活性不是Ni 2 P的固有性质。我们发现,在强电势下,在施加电势下会形成Ni氧化物/氢氧化物,从而提高了活性。此外,基于最近报道的Ni 2 P催化剂的电化学活性表面积的比活性不高,并且对于实际应用需要显着的改进。



























 京公网安备 11010802027423号
京公网安备 11010802027423号