Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Spatial Interactome Reveals the Protein Organization of the Algal CO2-Concentrating Mechanism.
Cell ( IF 64.5 ) Pub Date : 2017-Sep-21 , DOI: 10.1016/j.cell.2017.08.044 Luke C.M. Mackinder , Chris Chen , Ryan D. Leib , Weronika Patena , Sean R. Blum , Matthew Rodman , Silvia Ramundo , Christopher M. Adams , Martin C. Jonikas
Cell ( IF 64.5 ) Pub Date : 2017-Sep-21 , DOI: 10.1016/j.cell.2017.08.044 Luke C.M. Mackinder , Chris Chen , Ryan D. Leib , Weronika Patena , Sean R. Blum , Matthew Rodman , Silvia Ramundo , Christopher M. Adams , Martin C. Jonikas
|
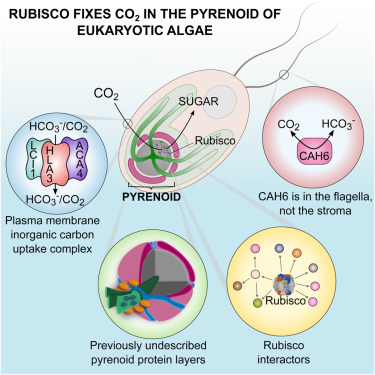
Approximately one-third of global CO2 fixation is performed by eukaryotic algae. Nearly all algae enhance their carbon assimilation by operating a CO2-concentrating mechanism (CCM) built around an organelle called the pyrenoid, whose protein composition is largely unknown. Here, we developed tools in the model alga Chlamydomonas reinhardtii to determine the localizations of 135 candidate CCM proteins and physical interactors of 38 of these proteins. Our data reveal the identity of 89 pyrenoid proteins, including Rubisco-interacting proteins, photosystem I assembly factor candidates, and inorganic carbon flux components. We identify three previously undescribed protein layers of the pyrenoid: a plate-like layer, a mesh layer, and a punctate layer. We find that the carbonic anhydrase CAH6 is in the flagella, not in the stroma that surrounds the pyrenoid as in current models. These results provide an overview of proteins operating in the eukaryotic algal CCM, a key process that drives global carbon fixation.
中文翻译:

空间交互作用揭示了藻类CO2浓缩机制的蛋白质组织。
真核藻完成了全球CO 2固定的大约三分之一。几乎所有藻类都通过运行CO 2来增强其碳同化作用浓缩机制(CCM)围绕着称为类胡萝卜素的细胞器而建立,其蛋白质组成在很大程度上未知。在这里,我们在海藻衣藻模型中开发了工具,以确定135个候选CCM蛋白的定位以及其中38个蛋白的物理相互作用体。我们的数据揭示了89种类胡萝卜素蛋白的身份,包括与Rubisco相互作用的蛋白,光系统I装配因子候选物和无机碳通量组分。我们确定了类胡萝卜素的三个以前未描述的蛋白质层:板状层,网状层和点状层。我们发现碳酸酐酶CAH6在鞭毛中,而不是在当前模型中围绕类胡萝卜素的基质中。这些结果概述了在真核藻类CCM中起作用的蛋白质,
更新日期:2017-09-21
中文翻译:

空间交互作用揭示了藻类CO2浓缩机制的蛋白质组织。
真核藻完成了全球CO 2固定的大约三分之一。几乎所有藻类都通过运行CO 2来增强其碳同化作用浓缩机制(CCM)围绕着称为类胡萝卜素的细胞器而建立,其蛋白质组成在很大程度上未知。在这里,我们在海藻衣藻模型中开发了工具,以确定135个候选CCM蛋白的定位以及其中38个蛋白的物理相互作用体。我们的数据揭示了89种类胡萝卜素蛋白的身份,包括与Rubisco相互作用的蛋白,光系统I装配因子候选物和无机碳通量组分。我们确定了类胡萝卜素的三个以前未描述的蛋白质层:板状层,网状层和点状层。我们发现碳酸酐酶CAH6在鞭毛中,而不是在当前模型中围绕类胡萝卜素的基质中。这些结果概述了在真核藻类CCM中起作用的蛋白质,


























 京公网安备 11010802027423号
京公网安备 11010802027423号