Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.tet.2017.09.038 Thomas Rosenau , Nele Sophie Zwirchmayr , Takashi Hosoya , Andreas Hofinger-Horvath , Markus Bacher , Antje Potthast
|
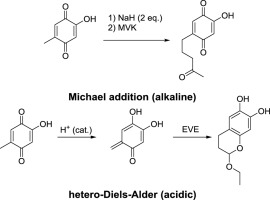
The 5-methyl group in 2-hydroxy-[1,4]-benzoquinone shows a peculiar reactivity that depends on the pH of the reaction medium. Under acidic conditions, ortho-quinone methide tautomers of the parent para-quinones are present in equilibrium. The 5-methylene group is stabilized by resonance, incorporating canonic structures with a positive charge at this position and now acting as carbenium equivalent. These ortho-quinone methide intermediates can be trapped in hetero-Diels-Alder reactions with inverse electron demand or by reaction with trimethylsilyl chloride. Under alkaline/basic conditions, the 5-methyl group is deprotonated, this time performing as carbanion equivalent being once more stabilized by resonance. This facet of reactivity was used for instance in regioselective perdeuteration of the 5-methyl position or in Michael additions. The reaction medium is thus able to govern the chemical behavior of the 5-methyl group, to effect its umpoling and to switch the reactivity to the opposite. Like the Roman god Janus, these methyl groups have two faces, and the reaction medium decides which one is presented.
中文翻译:

2-羟基-5-甲基-[1,4]-苯醌中的面向Janus的5-甲基
2-羟基-[1,4]-苯醌中的5-甲基显示出特定的反应性,该反应性取决于反应介质的pH。在酸性条件下,母体对-醌的邻-醌甲基化物互变异构体处于平衡状态。5-亚甲基通过共振得以稳定,并在该位置结合了带正电荷的正构结构,现在起了碳当量的作用。这些邻里苯醌甲基化物中间体可在逆电子需求或与三甲基甲硅烷基氯反应的情况下被捕获在杂Diels-Alder反应中。在碱性/碱性条件下,5-甲基被去质子化,这一次的碳负离子当量通过共振再次稳定下来。该反应性方面例如用于5-甲基位置的区域选择性氘代或迈克尔加成中。因此,反应介质能够控制5-甲基的化学行为,实现其本体化并将反应性改变为相反的。像罗马神贾纳斯(Janus)一样,这些甲基有两个面,反应介质决定了其中一个。


























 京公网安备 11010802027423号
京公网安备 11010802027423号