当前位置:
X-MOL 学术
›
N. Engl. J. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Fate of FDA Postapproval Studies
The New England Journal of Medicine ( IF 158.5 ) Pub Date : 2017-09-20 , DOI: 10.1056/nejmp1705800 Steven Woloshin , Lisa M. Schwartz , Brian White , Thomas J. Moore
The New England Journal of Medicine ( IF 158.5 ) Pub Date : 2017-09-20 , DOI: 10.1056/nejmp1705800 Steven Woloshin , Lisa M. Schwartz , Brian White , Thomas J. Moore
|
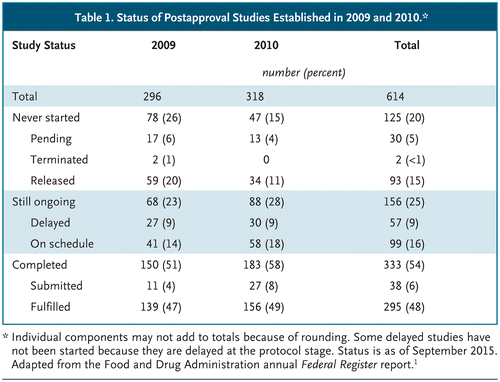
The Food and Drug Administration often requires postapproval studies to address issues such as optimal dosing, potential long-term side effects, and use in children or to confirm a drug’s clinical benefit. But many of these studies aren’t completed on time, if at all.
中文翻译:

FDA批准后研究的命运
美国食品药品监督管理局通常需要进行批准后研究,以解决诸如最佳剂量,潜在的长期副作用以及在儿童中使用等问题,或确认药物的临床益处。但是,其中许多研究甚至都没有按时完成。
更新日期:2017-09-20
中文翻译:

FDA批准后研究的命运
美国食品药品监督管理局通常需要进行批准后研究,以解决诸如最佳剂量,潜在的长期副作用以及在儿童中使用等问题,或确认药物的临床益处。但是,其中许多研究甚至都没有按时完成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号