当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Evidence for the Dopamine-First Mechanism of Norcoclaurine Synthase
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.biochem.7b00769 Benjamin R. Lichman 1 , Altin Sula 2 , Thomas Pesnot 3 , Helen C. Hailes 3 , John M. Ward 1 , Nicholas H. Keep 2
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.biochem.7b00769 Benjamin R. Lichman 1 , Altin Sula 2 , Thomas Pesnot 3 , Helen C. Hailes 3 , John M. Ward 1 , Nicholas H. Keep 2
Affiliation
|
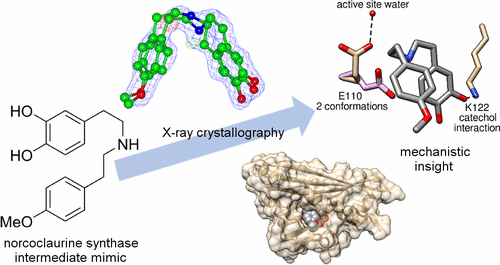
Norcoclaurine synthase (NCS) is a Pictet-Spenglerase that catalyzes the first key step in plant benzylisoquinoline alkaloid metabolism, a compound family that includes bioactive natural products such as morphine. The enzyme has also shown great potential as a biocatalyst for the formation of chiral isoquinolines. Here we present new high-resolution X-ray crystallography data describing Thalictrum flavum NCS bound to a mechanism-inspired ligand. The structure supports two key features of the NCS “dopamine-first” mechanism: the binding of dopamine catechol to Lys-122 and the position of the carbonyl substrate binding site at the active site entrance. The catalytically vital residue Glu-110 occupies a previously unobserved ligand-bound conformation that may be catalytically significant. The potential roles of inhibitory binding and alternative amino acid conformations in the mechanism have also been revealed. This work significantly advances our understanding of the NCS mechanism and will aid future efforts to engineer the substrate scope and catalytic properties of this useful biocatalyst.
中文翻译:

Norcoclaurine合酶的多巴胺第一机制的结构证据。
Norcoclaurine合酶(NCS)是Pictet-Spenglerase,它催化植物苄基异喹啉生物碱代谢中的第一个关键步骤,该化合物家族包括生物活性天然产物(例如吗啡)。该酶作为形成手性异喹啉的生物催化剂也显示出巨大的潜力。在这里,我们介绍了新的高分辨率X射线晶体学数据,描述了Thalictrum flavumNCS与受机制启发的配体结合。该结构支持NCS“多巴胺优先”机制的两个关键特征:多巴胺儿茶酚与Lys-122的结合以及羰基底物结合位点在活性位点入口处的位置。具有催化活性的残基Glu-110具有以前未观察到的配体结合构象,该构象可能具有催化作用。还已经揭示了抑制性结合和其他氨基酸构象在该机制中的潜在作用。这项工作大大提高了我们对NCS机理的理解,并将有助于未来的工作来设计这种有用生物催化剂的底物范围和催化性能。
更新日期:2017-09-20
中文翻译:

Norcoclaurine合酶的多巴胺第一机制的结构证据。
Norcoclaurine合酶(NCS)是Pictet-Spenglerase,它催化植物苄基异喹啉生物碱代谢中的第一个关键步骤,该化合物家族包括生物活性天然产物(例如吗啡)。该酶作为形成手性异喹啉的生物催化剂也显示出巨大的潜力。在这里,我们介绍了新的高分辨率X射线晶体学数据,描述了Thalictrum flavumNCS与受机制启发的配体结合。该结构支持NCS“多巴胺优先”机制的两个关键特征:多巴胺儿茶酚与Lys-122的结合以及羰基底物结合位点在活性位点入口处的位置。具有催化活性的残基Glu-110具有以前未观察到的配体结合构象,该构象可能具有催化作用。还已经揭示了抑制性结合和其他氨基酸构象在该机制中的潜在作用。这项工作大大提高了我们对NCS机理的理解,并将有助于未来的工作来设计这种有用生物催化剂的底物范围和催化性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号