当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-Selective Rhodium(III)-Catalyzed C−H Amination of 7-Azaindoles with Anthranils: Synthesis and Anticancer Evaluation
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-09-20 03:35:34 , DOI: 10.1002/adsc.201700800 Mijin Jeon 1 , Jihye Park 1 , Prasanta Dey 1 , Yongguk Oh 1 , Hyunjung Oh 1 , Sangil Han 1 , Sung Hee Um 2 , Hyung Sik Kim 1 , Neeraj Kumar Mishra 1 , In Su Kim 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-09-20 03:35:34 , DOI: 10.1002/adsc.201700800 Mijin Jeon 1 , Jihye Park 1 , Prasanta Dey 1 , Yongguk Oh 1 , Hyunjung Oh 1 , Sangil Han 1 , Sung Hee Um 2 , Hyung Sik Kim 1 , Neeraj Kumar Mishra 1 , In Su Kim 1
Affiliation
|
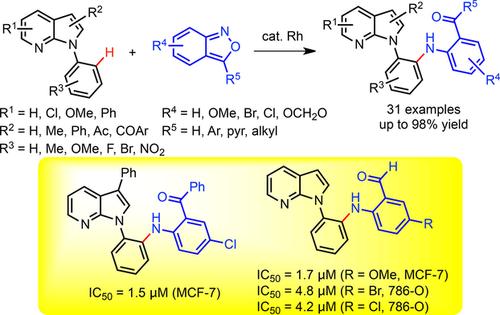
The site-selective C−H amination reaction of 7-azaindoles with various benzisoxazoles as amination surrogates under cationic rhodium(III) catalysis is described. This transformation efficiently provides a range of ortho-aminated N-aryl-7-azaindoles with excellent site-selectivity and functional group compatibility. The formed ortho-aminated 7-azaindoles were readily transformed into biologically relevant heterocycles such as azaindoloacridine, azaindoloacridone, and bis-indole compounds. Moreover, the synthetic derivatives were tested for in vitro anticancer activity against human breast adenocarcinoma cells (MCF-7), human renal carcinoma cells (786-O), and human prostate adenocarcinoma cells (DU145). Notably, some synthetic compounds were found to display most potent anticancer activity, compared to that of anticancer doxorubicin as a positive control.
中文翻译:

位置选择性铑(III)催化的7-氮杂吲哚与蒽的CH氨基化反应:合成和抗癌性评估
描述了在阳离子铑(III)催化下7-氮杂吲哚与各种苯并异恶唑作为胺代用品的定点CH胺化反应。该转化有效地提供了一系列具有优异的位点选择性和官能团相容性的邻氨基化的N-芳基-7-氮杂吲哚。形成的邻氨基化的7-氮杂吲哚易于转化成生物学上相关的杂环,例如氮杂吲哚并r啶,氮杂吲哚并rid啶酮和双吲哚化合物。此外,还对合成衍生物进行了体外测试。对人乳腺腺癌细胞(MCF-7),人肾癌细胞(786-O)和人前列腺腺癌细胞(DU145)的抗癌活性。值得注意的是,与抗癌阿霉素作为阳性对照相比,发现某些合成化合物显示出最有效的抗癌活性。
更新日期:2017-09-20
中文翻译:

位置选择性铑(III)催化的7-氮杂吲哚与蒽的CH氨基化反应:合成和抗癌性评估
描述了在阳离子铑(III)催化下7-氮杂吲哚与各种苯并异恶唑作为胺代用品的定点CH胺化反应。该转化有效地提供了一系列具有优异的位点选择性和官能团相容性的邻氨基化的N-芳基-7-氮杂吲哚。形成的邻氨基化的7-氮杂吲哚易于转化成生物学上相关的杂环,例如氮杂吲哚并r啶,氮杂吲哚并rid啶酮和双吲哚化合物。此外,还对合成衍生物进行了体外测试。对人乳腺腺癌细胞(MCF-7),人肾癌细胞(786-O)和人前列腺腺癌细胞(DU145)的抗癌活性。值得注意的是,与抗癌阿霉素作为阳性对照相比,发现某些合成化合物显示出最有效的抗癌活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号