当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-aminoBODIPYs by palladium catalysed amination
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1039/c7ob01767g Rua B. Alnoman 1, 2, 3, 4 , Patrycja Stachelek 1, 2, 3, 4 , Julian G. Knight 1, 2, 3, 4 , Anthony Harriman 1, 2, 3, 4 , Paul G. Waddell 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1039/c7ob01767g Rua B. Alnoman 1, 2, 3, 4 , Patrycja Stachelek 1, 2, 3, 4 , Julian G. Knight 1, 2, 3, 4 , Anthony Harriman 1, 2, 3, 4 , Paul G. Waddell 1, 2, 3, 4
Affiliation
|
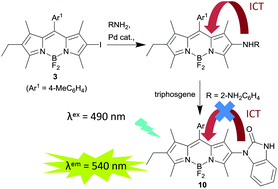
Palladium catalysed coupling of the 2-iodoBODIPY 3 with a range of anilines and a primary alkylamine succeeds in generating the corresponding 2-aminoBODIPYs. These 2-aminoBODIPY derivatives are non-emissive and quantum chemical calculations and electrochemistry are consistent with charge transfer from the amine substituent. Attenuation of this charge transfer pathway by conversion of the 1,2-phenylenediamine derivative 9 into the corresponding benzimidazolone 10 restores the fluorescence and has been used as the basis for a fluorescence sensor for phosgene.
中文翻译:

钯催化胺化合成2-aminoBODIPYs
钯催化的2-ioBODIPY 3与一系列苯胺和伯烷基胺的偶联成功地产生了相应的2-aminoBODIPY。这些2-aminoBODIPY衍生物是非发射性的,并且量子化学计算和电化学与胺取代基的电荷转移是一致的。通过将1,2-苯二胺衍生物9转化为相应的苯并咪唑酮10来减弱该电荷转移途径,从而恢复了荧光,并已被用作光气荧光传感器的基础。
更新日期:2017-09-20
中文翻译:

钯催化胺化合成2-aminoBODIPYs
钯催化的2-ioBODIPY 3与一系列苯胺和伯烷基胺的偶联成功地产生了相应的2-aminoBODIPY。这些2-aminoBODIPY衍生物是非发射性的,并且量子化学计算和电化学与胺取代基的电荷转移是一致的。通过将1,2-苯二胺衍生物9转化为相应的苯并咪唑酮10来减弱该电荷转移途径,从而恢复了荧光,并已被用作光气荧光传感器的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号