当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
1,5-Electrocyclization of conjugated azomethine ylides derived from 3-formyl chromene and N-alkyl amino acids/esters
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7ob00705a E. Pravardhan Reddy 1, 2, 3, 4 , A. Sumankumar 4, 5, 6, 7 , B. Sridhar 4, 6, 8, 9 , Y. Hemasri 1, 2, 4, 10 , Y. Jayaprakash Rao 1, 1, 2, 3, 4 , B. V. Subba Reddy 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7ob00705a E. Pravardhan Reddy 1, 2, 3, 4 , A. Sumankumar 4, 5, 6, 7 , B. Sridhar 4, 6, 8, 9 , Y. Hemasri 1, 2, 4, 10 , Y. Jayaprakash Rao 1, 1, 2, 3, 4 , B. V. Subba Reddy 4, 5, 6, 7
Affiliation
|
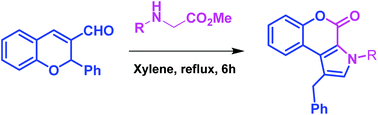
A novel strategy has been developed for the synthesis of chromeno[3,4-b]pyrrol-4(3H)-one and substituted pyrrole derivatives through 1,5-electrocyclization of conjugated azomethine ylides. This is the first example of the preparation of highly substituted pyrrole derivatives from chromene-3-carboxaldehydes (non-enolizable aldehydes) and N-alkyl amino acids/esters. This method is simple and applicable to a diverse range of substrates.
中文翻译:

衍生自3-甲酰基亚铬基和N-烷基氨基酸/酯的共轭甲亚胺基的1,5-环电化
已开发出一种新的策略,用于通过共轭偶氮甲亚胺的1,5-电环化合成chromeno [3,4- b ] pyrrol-4(3 H)-one和取代的吡咯衍生物。这是由亚甲基-3-羧醛(不可烯化的醛)和N-烷基氨基酸/酯制备高度取代的吡咯衍生物的第一个例子。该方法很简单,适用于各种基材。
更新日期:2017-09-20
中文翻译:

衍生自3-甲酰基亚铬基和N-烷基氨基酸/酯的共轭甲亚胺基的1,5-环电化
已开发出一种新的策略,用于通过共轭偶氮甲亚胺的1,5-电环化合成chromeno [3,4- b ] pyrrol-4(3 H)-one和取代的吡咯衍生物。这是由亚甲基-3-羧醛(不可烯化的醛)和N-烷基氨基酸/酯制备高度取代的吡咯衍生物的第一个例子。该方法很简单,适用于各种基材。



























 京公网安备 11010802027423号
京公网安备 11010802027423号