当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electronic effects on a one-pot aromatization cascade involving alkynyl-Prins cyclization, Friedel–Crafts alkylation and dehydration to tricyclic benzo[f]isochromenes
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7ob01412k Robert J. Hinkle 1, 2, 3, 4 , Yuzhou Chen 1, 2, 3, 4 , Colleen P. Nofi 1, 2, 3, 4 , Shane E. Lewis 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7ob01412k Robert J. Hinkle 1, 2, 3, 4 , Yuzhou Chen 1, 2, 3, 4 , Colleen P. Nofi 1, 2, 3, 4 , Shane E. Lewis 1, 2, 3, 4
Affiliation
|
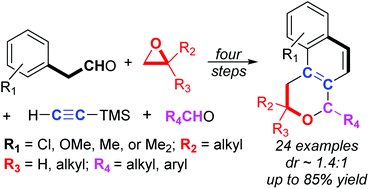
A three-step domino reaction between 1-aryl-3-hexyne-2,6-diol derivatives and aldehydes is used to construct tricyclic 1,4-dihydro-2H-benzo[f]isochromenes. The cascade is initiated by BF3·OEt2 and involves alkynyl-Prins cyclization, Friedel–Crafts alkenylation, and dehydration/aromatization to create a new, central aromatic ring and eliminate 2 equiv. of water. Electron-donating substituents on the aryl ring of the 1-aryl-3-hexyne-2,6-diols significantly increase overall yields as do electron-rich aldehyde reaction partners. For 2,4-disubstituted 2H-benzo[f]isochromene products, diastereoselectivities in the alkynyl-Prins reaction are ∼1.4 :
: 1 in favor of the cis-diastereomer. The stereochemistry of one cis-product was verified by X-ray crystallographic analysis and a second structure was also verified by X-ray analysis.
1 in favor of the cis-diastereomer. The stereochemistry of one cis-product was verified by X-ray crystallographic analysis and a second structure was also verified by X-ray analysis.
中文翻译:

一锅芳构化级联反应的电子效应,涉及炔基-Prins环化,Friedel-Crafts烷基化和脱水成三环苯并[ f ]异色酮
1-芳基-3-己炔-2,6-二醇衍生物与醛之间的三步多米诺反应用于构建三环1,4-二氢-2 H-苯并[ f ]异色酮。级联反应是由BF 3 ·OEt 2引发的,涉及炔基-Prins环化,Friedel-Crafts烯基化和脱水/芳构化,以形成一个新的中心芳环并消除2个当量。水。1-芳基-3-己炔-2,6-二醇芳基环上的供电子取代基与富电子醛反应伙伴一样,可显着提高总收率。对于2,4-二取代的2 H-苯并[ f ]异戊二烯产物,炔基-普林斯反应中的非对映选择性为〜1.4 :
: 1表示顺式-非对映异构体。X射线晶体学分析证实了一种顺式产物的立体化学,X射线分析也证实了第二种结构的立体化学。
1表示顺式-非对映异构体。X射线晶体学分析证实了一种顺式产物的立体化学,X射线分析也证实了第二种结构的立体化学。
更新日期:2017-09-20
 :
: 1 in favor of the cis-diastereomer. The stereochemistry of one cis-product was verified by X-ray crystallographic analysis and a second structure was also verified by X-ray analysis.
1 in favor of the cis-diastereomer. The stereochemistry of one cis-product was verified by X-ray crystallographic analysis and a second structure was also verified by X-ray analysis.
中文翻译:

一锅芳构化级联反应的电子效应,涉及炔基-Prins环化,Friedel-Crafts烷基化和脱水成三环苯并[ f ]异色酮
1-芳基-3-己炔-2,6-二醇衍生物与醛之间的三步多米诺反应用于构建三环1,4-二氢-2 H-苯并[ f ]异色酮。级联反应是由BF 3 ·OEt 2引发的,涉及炔基-Prins环化,Friedel-Crafts烯基化和脱水/芳构化,以形成一个新的中心芳环并消除2个当量。水。1-芳基-3-己炔-2,6-二醇芳基环上的供电子取代基与富电子醛反应伙伴一样,可显着提高总收率。对于2,4-二取代的2 H-苯并[ f ]异戊二烯产物,炔基-普林斯反应中的非对映选择性为〜1.4
 :
: 1表示顺式-非对映异构体。X射线晶体学分析证实了一种顺式产物的立体化学,X射线分析也证实了第二种结构的立体化学。
1表示顺式-非对映异构体。X射线晶体学分析证实了一种顺式产物的立体化学,X射线分析也证实了第二种结构的立体化学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号