当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Long-term protein packaging in cholinium-based ionic liquids: improved catalytic activity and enhanced stability of cytochrome c against multiple stresses
Green Chemistry ( IF 9.8 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1039/c7gc02011b Meena Bisht 1, 2, 3, 4, 5 , Dibyendu Mondal 5, 6, 7, 8, 9 , Matheus M. Pereira 5, 6, 7, 8, 9 , Mara G. Freire 5, 6, 7, 8, 9 , P. Venkatesu 1, 2, 3, 4 , J. A. P. Coutinho 5, 6, 7, 8, 9
Green Chemistry ( IF 9.8 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1039/c7gc02011b Meena Bisht 1, 2, 3, 4, 5 , Dibyendu Mondal 5, 6, 7, 8, 9 , Matheus M. Pereira 5, 6, 7, 8, 9 , Mara G. Freire 5, 6, 7, 8, 9 , P. Venkatesu 1, 2, 3, 4 , J. A. P. Coutinho 5, 6, 7, 8, 9
Affiliation
|
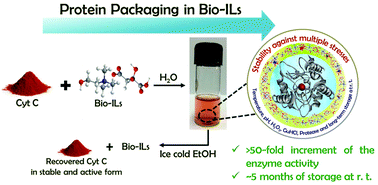
There is considerable interest in the use of structurally stable and catalytically active enzymes, such as cytochrome c (Cyt c), in the pharmaceutical and fine chemicals industries. However, harsh process conditions, such as temperature, pH, and the presence of organic solvents, are the major barrier to the effective use of enzymes in biocatalysis. We demonstrate the suitability of cholinium-based ionic liquids (ILs) formed by the cholinium cation and dicarboxylate-based anions as potential media for enzymes, in which remarkable enhanced activity and improved stability of Cyt c against multiple stresses were obtained. Among the several ILs studied, an exceptionally high catalytic activity (>50-fold) of Cyt c was observed in the aqueous solutions of cholinium glutarate ([Ch][Glu]; at 1 :
: 1 weight ratio of IL
1 weight ratio of IL :
: H2O) compared to the commonly used phosphate buffer solutions (pH 7.2), and >25-fold compared to the aqueous solutions of cholinium dihydrogen phosphate ([Ch][Dhp]; 1
H2O) compared to the commonly used phosphate buffer solutions (pH 7.2), and >25-fold compared to the aqueous solutions of cholinium dihydrogen phosphate ([Ch][Dhp]; 1 :
: 2 weight ratio of IL
2 weight ratio of IL :
: water)—the best known IL for long term stability of Cyt c. The catalytic activity of the enzyme in the presence of ILs was retained against several external stimuli, such as chemical denaturants (H2O2 and GuHCl) and temperatures of up to 120 °C. The observed enzyme activity is in agreement with its structural stability, as confirmed by UV–vis, circular dichroism (CD), and Fourier transform infrared (FT-IR) spectroscopy. Taking advantage of the multi-ionization states of di/tri-carboxylic acids, the pH was switched from acidic to basic by the addition of the corresponding carboxylic acid and choline hydroxide, respectively. The activity was found to be maximum at a 1
water)—the best known IL for long term stability of Cyt c. The catalytic activity of the enzyme in the presence of ILs was retained against several external stimuli, such as chemical denaturants (H2O2 and GuHCl) and temperatures of up to 120 °C. The observed enzyme activity is in agreement with its structural stability, as confirmed by UV–vis, circular dichroism (CD), and Fourier transform infrared (FT-IR) spectroscopy. Taking advantage of the multi-ionization states of di/tri-carboxylic acids, the pH was switched from acidic to basic by the addition of the corresponding carboxylic acid and choline hydroxide, respectively. The activity was found to be maximum at a 1 :
: 1 molar ratio of [Ch][carboxylate], with a pH in the range from 3 to 5.5. Moreover, it was found that the cholinium-based ILs studied herein protect the enzyme against protease digestion and allow long-term storage (at least for 21 weeks) at room temperature. An attempt by molecular docking was also made to better understand the efficacy of the investigated cholinium-based ILs towards the enhanced activity and long term stability of Cyt c. The results showed that dicarboxylate anions interact with the active site's amino acids of the enzyme through H-bonding and electrostatic interactions, which are responsible for the observed enhancement of the catalytic activity. Finally, it is demonstrated that Cyt c can be successfully recovered from the aqueous solution of ILs and reused without compromising its yield, structural integrity and catalytic activity, thereby overcoming the major limitations in the use of IL–protein systems in biocatalysis.
1 molar ratio of [Ch][carboxylate], with a pH in the range from 3 to 5.5. Moreover, it was found that the cholinium-based ILs studied herein protect the enzyme against protease digestion and allow long-term storage (at least for 21 weeks) at room temperature. An attempt by molecular docking was also made to better understand the efficacy of the investigated cholinium-based ILs towards the enhanced activity and long term stability of Cyt c. The results showed that dicarboxylate anions interact with the active site's amino acids of the enzyme through H-bonding and electrostatic interactions, which are responsible for the observed enhancement of the catalytic activity. Finally, it is demonstrated that Cyt c can be successfully recovered from the aqueous solution of ILs and reused without compromising its yield, structural integrity and catalytic activity, thereby overcoming the major limitations in the use of IL–protein systems in biocatalysis.
中文翻译:

在基于胆碱的离子液体中的长期蛋白质包装:改善的催化活性和增强的细胞色素c抵抗多种压力的稳定性
在制药和精细化工行业中,使用结构稳定且具有催化活性的酶(例如细胞色素c(Cyt c))引起了极大的兴趣。然而,苛刻的工艺条件,例如温度,pH值和有机溶剂的存在,是在生物催化中有效使用酶的主要障碍。我们证明了由胆碱阳离子和基于二羧酸根的阴离子形成的基于胆碱的离子液体(ILs)作为酶的潜在媒介的适宜性,其中获得了显着增强的活性和Cyt c抵抗多种压力的稳定性。在研究的几种IL中,在谷氨酸胆碱([Ch] [Glu]; IL的重量比为1 :
: 1)的水溶液中观察到Cyt c的异常高的催化活性(> 50倍)
1)的水溶液中观察到Cyt c的异常高的催化活性(> 50倍) :
: ħ 2 O)相比,常用的磷酸盐缓冲溶液(pH 7.2),和> 25倍相比磷酸胆碱二氢水溶液([CH] [DHP]; 1
ħ 2 O)相比,常用的磷酸盐缓冲溶液(pH 7.2),和> 25倍相比磷酸胆碱二氢水溶液([CH] [DHP]; 1  :
: IL的2重量比
IL的2重量比 :
: 水) -Cyt c的长期稳定性最著名的IL。在白介素存在下,该酶的催化活性得以保留,可抵抗多种外部刺激,例如化学变性剂(H 2 O 2和GuHCl)和最高120°C的温度。紫外可见,圆二色性(CD)和傅立叶变换红外(FT-IR)光谱证实了所观察到的酶活性与其结构稳定性相符。利用二/三羧酸的多电离状态,分别通过添加相应的羧酸和氢氧化胆碱将pH从酸性切换为碱性。发现该活性最大为1
水) -Cyt c的长期稳定性最著名的IL。在白介素存在下,该酶的催化活性得以保留,可抵抗多种外部刺激,例如化学变性剂(H 2 O 2和GuHCl)和最高120°C的温度。紫外可见,圆二色性(CD)和傅立叶变换红外(FT-IR)光谱证实了所观察到的酶活性与其结构稳定性相符。利用二/三羧酸的多电离状态,分别通过添加相应的羧酸和氢氧化胆碱将pH从酸性切换为碱性。发现该活性最大为1  :
: [Ch] [羧酸盐]的摩尔比为1,pH在3至5.5的范围内。此外,发现本文研究的基于胆碱的IL保护酶免受蛋白酶消化,并允许在室温下长期保存(至少21周)。还尝试通过分子对接来更好地理解所研究的基于胆碱的IL对Cyt c的增强的活性和长期稳定性的功效。结果表明,二羧酸根阴离子通过H键和静电相互作用与酶的活性位点氨基酸相互作用,这是观察到的催化活性增强的原因。最后,证明Cyt c可以成功地从IL的水溶液中回收并重新使用,而不会影响其收率,
[Ch] [羧酸盐]的摩尔比为1,pH在3至5.5的范围内。此外,发现本文研究的基于胆碱的IL保护酶免受蛋白酶消化,并允许在室温下长期保存(至少21周)。还尝试通过分子对接来更好地理解所研究的基于胆碱的IL对Cyt c的增强的活性和长期稳定性的功效。结果表明,二羧酸根阴离子通过H键和静电相互作用与酶的活性位点氨基酸相互作用,这是观察到的催化活性增强的原因。最后,证明Cyt c可以成功地从IL的水溶液中回收并重新使用,而不会影响其收率,
更新日期:2017-09-20
 :
: 1 weight ratio of IL
1 weight ratio of IL :
: H2O) compared to the commonly used phosphate buffer solutions (pH 7.2), and >25-fold compared to the aqueous solutions of cholinium dihydrogen phosphate ([Ch][Dhp]; 1
H2O) compared to the commonly used phosphate buffer solutions (pH 7.2), and >25-fold compared to the aqueous solutions of cholinium dihydrogen phosphate ([Ch][Dhp]; 1 :
: 2 weight ratio of IL
2 weight ratio of IL :
: water)—the best known IL for long term stability of Cyt c. The catalytic activity of the enzyme in the presence of ILs was retained against several external stimuli, such as chemical denaturants (H2O2 and GuHCl) and temperatures of up to 120 °C. The observed enzyme activity is in agreement with its structural stability, as confirmed by UV–vis, circular dichroism (CD), and Fourier transform infrared (FT-IR) spectroscopy. Taking advantage of the multi-ionization states of di/tri-carboxylic acids, the pH was switched from acidic to basic by the addition of the corresponding carboxylic acid and choline hydroxide, respectively. The activity was found to be maximum at a 1
water)—the best known IL for long term stability of Cyt c. The catalytic activity of the enzyme in the presence of ILs was retained against several external stimuli, such as chemical denaturants (H2O2 and GuHCl) and temperatures of up to 120 °C. The observed enzyme activity is in agreement with its structural stability, as confirmed by UV–vis, circular dichroism (CD), and Fourier transform infrared (FT-IR) spectroscopy. Taking advantage of the multi-ionization states of di/tri-carboxylic acids, the pH was switched from acidic to basic by the addition of the corresponding carboxylic acid and choline hydroxide, respectively. The activity was found to be maximum at a 1 :
: 1 molar ratio of [Ch][carboxylate], with a pH in the range from 3 to 5.5. Moreover, it was found that the cholinium-based ILs studied herein protect the enzyme against protease digestion and allow long-term storage (at least for 21 weeks) at room temperature. An attempt by molecular docking was also made to better understand the efficacy of the investigated cholinium-based ILs towards the enhanced activity and long term stability of Cyt c. The results showed that dicarboxylate anions interact with the active site's amino acids of the enzyme through H-bonding and electrostatic interactions, which are responsible for the observed enhancement of the catalytic activity. Finally, it is demonstrated that Cyt c can be successfully recovered from the aqueous solution of ILs and reused without compromising its yield, structural integrity and catalytic activity, thereby overcoming the major limitations in the use of IL–protein systems in biocatalysis.
1 molar ratio of [Ch][carboxylate], with a pH in the range from 3 to 5.5. Moreover, it was found that the cholinium-based ILs studied herein protect the enzyme against protease digestion and allow long-term storage (at least for 21 weeks) at room temperature. An attempt by molecular docking was also made to better understand the efficacy of the investigated cholinium-based ILs towards the enhanced activity and long term stability of Cyt c. The results showed that dicarboxylate anions interact with the active site's amino acids of the enzyme through H-bonding and electrostatic interactions, which are responsible for the observed enhancement of the catalytic activity. Finally, it is demonstrated that Cyt c can be successfully recovered from the aqueous solution of ILs and reused without compromising its yield, structural integrity and catalytic activity, thereby overcoming the major limitations in the use of IL–protein systems in biocatalysis.
中文翻译:

在基于胆碱的离子液体中的长期蛋白质包装:改善的催化活性和增强的细胞色素c抵抗多种压力的稳定性
在制药和精细化工行业中,使用结构稳定且具有催化活性的酶(例如细胞色素c(Cyt c))引起了极大的兴趣。然而,苛刻的工艺条件,例如温度,pH值和有机溶剂的存在,是在生物催化中有效使用酶的主要障碍。我们证明了由胆碱阳离子和基于二羧酸根的阴离子形成的基于胆碱的离子液体(ILs)作为酶的潜在媒介的适宜性,其中获得了显着增强的活性和Cyt c抵抗多种压力的稳定性。在研究的几种IL中,在谷氨酸胆碱([Ch] [Glu]; IL的重量比为1
 :
: 1)的水溶液中观察到Cyt c的异常高的催化活性(> 50倍)
1)的水溶液中观察到Cyt c的异常高的催化活性(> 50倍) :
: ħ 2 O)相比,常用的磷酸盐缓冲溶液(pH 7.2),和> 25倍相比磷酸胆碱二氢水溶液([CH] [DHP]; 1
ħ 2 O)相比,常用的磷酸盐缓冲溶液(pH 7.2),和> 25倍相比磷酸胆碱二氢水溶液([CH] [DHP]; 1  :
: IL的2重量比
IL的2重量比 :
: 水) -Cyt c的长期稳定性最著名的IL。在白介素存在下,该酶的催化活性得以保留,可抵抗多种外部刺激,例如化学变性剂(H 2 O 2和GuHCl)和最高120°C的温度。紫外可见,圆二色性(CD)和傅立叶变换红外(FT-IR)光谱证实了所观察到的酶活性与其结构稳定性相符。利用二/三羧酸的多电离状态,分别通过添加相应的羧酸和氢氧化胆碱将pH从酸性切换为碱性。发现该活性最大为1
水) -Cyt c的长期稳定性最著名的IL。在白介素存在下,该酶的催化活性得以保留,可抵抗多种外部刺激,例如化学变性剂(H 2 O 2和GuHCl)和最高120°C的温度。紫外可见,圆二色性(CD)和傅立叶变换红外(FT-IR)光谱证实了所观察到的酶活性与其结构稳定性相符。利用二/三羧酸的多电离状态,分别通过添加相应的羧酸和氢氧化胆碱将pH从酸性切换为碱性。发现该活性最大为1  :
: [Ch] [羧酸盐]的摩尔比为1,pH在3至5.5的范围内。此外,发现本文研究的基于胆碱的IL保护酶免受蛋白酶消化,并允许在室温下长期保存(至少21周)。还尝试通过分子对接来更好地理解所研究的基于胆碱的IL对Cyt c的增强的活性和长期稳定性的功效。结果表明,二羧酸根阴离子通过H键和静电相互作用与酶的活性位点氨基酸相互作用,这是观察到的催化活性增强的原因。最后,证明Cyt c可以成功地从IL的水溶液中回收并重新使用,而不会影响其收率,
[Ch] [羧酸盐]的摩尔比为1,pH在3至5.5的范围内。此外,发现本文研究的基于胆碱的IL保护酶免受蛋白酶消化,并允许在室温下长期保存(至少21周)。还尝试通过分子对接来更好地理解所研究的基于胆碱的IL对Cyt c的增强的活性和长期稳定性的功效。结果表明,二羧酸根阴离子通过H键和静电相互作用与酶的活性位点氨基酸相互作用,这是观察到的催化活性增强的原因。最后,证明Cyt c可以成功地从IL的水溶液中回收并重新使用,而不会影响其收率,


























 京公网安备 11010802027423号
京公网安备 11010802027423号