当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cooperative hydrogen bonds form a pseudocycle stabilizing an isolated complex of isocyanic acid with urea
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1039/c7cp04315e John C. Mullaney 1, 2, 3, 4, 5 , Chris Medcraft 1, 2, 3, 4, 5 , David P. Tew 1, 5, 6, 7 , Luke Lewis-Borrell 1, 2, 3, 4, 5 , Bernard T. Golding 1, 2, 3, 4, 5 , Nicholas R. Walker 1, 2, 3, 4, 5 , Anthony C. Legon 1, 5, 6, 7
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1039/c7cp04315e John C. Mullaney 1, 2, 3, 4, 5 , Chris Medcraft 1, 2, 3, 4, 5 , David P. Tew 1, 5, 6, 7 , Luke Lewis-Borrell 1, 2, 3, 4, 5 , Bernard T. Golding 1, 2, 3, 4, 5 , Nicholas R. Walker 1, 2, 3, 4, 5 , Anthony C. Legon 1, 5, 6, 7
Affiliation
|
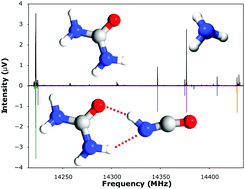
The shapes of macromolecules and their complexes with small molecules are often determined by extended networks of hydrogen bonds. Here, for the first time, we provide a detailed description of a cooperative pair of hydrogen bonds to an individual molecule of urea. The structure and properties of a gas phase complex formed between urea and isocyanic acid are characterised through microwave spectroscopy and ab initio calculations at the CCSD(T)(F12*)/aug-cc-pVTZ level.
中文翻译:

协同氢键形成伪环,稳定异氰酸与尿素的孤立配合物
大分子的形状及其与小分子的复合物通常由氢键的扩展网络决定。在这里,我们首次提供了与单个尿素分子的氢键配合对的详细描述。通过微波光谱和从头算在CCSD(T)(F12 *)/ aug-cc-pVTZ水平上对尿素和异氰酸之间形成的气相络合物的结构和性质进行了表征。
更新日期:2017-09-20
中文翻译:

协同氢键形成伪环,稳定异氰酸与尿素的孤立配合物
大分子的形状及其与小分子的复合物通常由氢键的扩展网络决定。在这里,我们首次提供了与单个尿素分子的氢键配合对的详细描述。通过微波光谱和从头算在CCSD(T)(F12 *)/ aug-cc-pVTZ水平上对尿素和异氰酸之间形成的气相络合物的结构和性质进行了表征。


























 京公网安备 11010802027423号
京公网安备 11010802027423号