Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.bmc.2017.09.028 Galina F. Makhaeva , Sofya V. Lushchekina , Natalia P. Boltneva , Olga G. Serebryakova , Elena V. Rudakova , Alexey A. Ustyugov , Sergey O. Bachurin , Alexander V. Shchepochkin , Oleg N. Chupakhin , Valery N. Charushin , Rudy J. Richardson
|
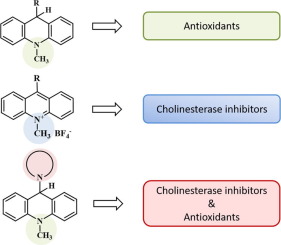
We investigated the inhibitory activity of 4 groups of novel acridine derivatives against acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and carboxylesterase (CaE) using the methods of enzyme kinetics and molecular docking. Antioxidant activity of the compounds was determined using the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) radical decolorization assay as their ability to scavenge free radicals. Analysis of the esterase profiles and antiradical activities of the acridine derivatives showed that 9-aryl(heteroaryl)-N-methyl-9,10-dihydroacridines have a high radical-scavenging activity but low potency as AChE and BChE inhibitors, whereas 9-aryl(heteroaryl)-N-methyl-acridinium tetrafluoroborates effectively inhibit cholinesterases but do not exhibit antiradical activity. In contrast, a group of derivatives of 9-heterocyclic amino-N-methyl-9,10-dihydroacridine has been found that combine effective inhibition of AChE and BChE with rather high radical-scavenging activity. The results of molecular docking well explain the observed features in the efficacy, selectivity, and mechanism of cholinesterase inhibition by the acridine derivatives. Thus, in a series of acridine derivatives we have found compounds possessing dual properties of effective and selective cholinesterase inhibition together with free radical scavenging, which makes promising the use of the acridine scaffold to create multifunctional drugs for the therapy of neurodegenerative diseases.
中文翻译:

具有9种取代基的cr啶衍生物,如乙酰胆碱酯酶和丁酰胆碱酯酶抑制剂,具有抗阿尔茨海默氏病的作用
我们使用酶动力学和分子对接的方法研究了4组新型a啶衍生物对乙酰胆碱酯酶(AChE),丁酰胆碱酯酶(BChE)和羧酸酯酶(CaE)的抑制活性。使用2,2'-叠氮基双-(3-乙基苯并噻唑啉-6-磺酸)(ABTS +)自由基脱色测定法测定化合物的抗氧化活性,以其清除自由基的能力。对ester啶衍生物的酯酶谱和抗自由基活性的分析表明,9-芳基(杂芳基)-N-甲基-9,10-二氢ac啶具有较高的自由基清除活性,但作为AChE和BChE抑制剂的效力较低,而9-芳基(杂芳基)-N-甲基-基四氟硼酸盐可有效抑制胆碱酯酶,但不表现出抗自由基活性。相反,已经发现一组9-杂环氨基-N-甲基-9,10-二氢ac啶的衍生物,其结合了对AChE和BChE的有效抑制以及相当高的自由基清除活性。分子对接的结果很好地解释了observed啶衍生物抑制胆碱酯酶的功效,选择性和机理中观察到的特征。因此,在一系列of啶衍生物中,我们发现了具有有效和选择性抑制胆碱酯酶抑制以及清除自由基的双重性质的化合物,这使得promising啶支架有望用于制造用于治疗神经退行性疾病的多功能药物。

























 京公网安备 11010802027423号
京公网安备 11010802027423号