Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.apcata.2017.09.021 Mengjie Pu , Zeyu Guan , Yongwen Ma , Jinquan Wan , Yan Wang , Mark L. Brusseau , Haiyuan Chi
|
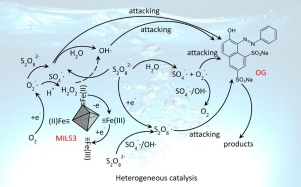
A series of MIL-53(Fe) materials was synthesized using a solvothermal method under different temperature and time conditions and were used as catalysts to activate persulfate and degrade Orange G (OG). Influences of the above conditions on the crystal structure and catalytic behavior were investigated. Degradation of OG under different conditions was evaluated, and the possible activation mechanism was speculated. The results indicate that high synthesis temperature (larger than 170 °C) leads to poor crystallinity and low catalytic activity, while MIL-53(Fe) cannot fully develop at low temperature (100 or 120 °C). The extension of synthesis time from 5 h to 3 d can increase the crystallinity of the samples, but weakened the catalytic activity, which was caused by the reduction of BET surface area and the amount of Fe (II)-coordinative unsaturated sites. Among all the samples, MIL-53(Fe)-A possesses the best crystal structure and catalytic activity. In optimal conditions, OG solution can be totally decolorized after degradation for 90 min, and a removal rate of 74% for COD was attained after 120 min. The initial solution pH had great influence on OG degradation, with the greatest removal in acidic pH environment. ESR spectra showed that sulfate radical (SO4−), hydroxyl radical (OH
), persulfate radical (S2O8−
), and superoxide radical (O2
) exist in this system under acidic conditions. Furthermore, with the increase of pH, the relative amount of O2
increases while that of OH
and SO4−
decreases, resulting in a reduced oxidizing capacity of the system.
中文翻译:

铁基金属有机骨架MIL-53的合成作为活化过硫酸盐以降解水溶液中橙G的有效催化剂
使用溶剂热法在不同的温度和时间条件下合成了一系列MIL-53(Fe)材料,并用作活化过硫酸盐和降解橙G(OG)的催化剂。研究了上述条件对晶体结构和催化性能的影响。评价了在不同条件下OG的降解,并推测了可能的活化机理。结果表明,较高的合成温度(大于170°C)会导致结晶度差和催化活性低,而MIL-53(Fe)在低温(100或120°C)下无法完全生成。合成时间从5 h延长到3 d可以提高样品的结晶度,但会削弱催化活性,这是由于BET表面积的减少和Fe(II)配位不饱和位点的数量减少所致。在所有样品中,MIL-53(Fe)-A具有最佳的晶体结构和催化活性。在最佳条件下,降解90分钟后OG溶液即可完全脱色,120分钟后OD溶液的COD去除率达到74%。初始溶液的pH值对OG的降解影响很大,而在酸性pH值环境中去除率最大。ESR谱显示硫酸根(SO 在酸性pH环境中具有最大的去除率。ESR谱显示硫酸根(SO 在酸性pH环境中具有最大的去除率。ESR谱显示硫酸根(SO4 - ),羟基自由基(OH
),过硫酸盐自由基(š 2 ö 8 -
)和超氧自由基(O 2
)在酸性条件下与该系统中存在。此外,随着pH值的升高,的O-相对量2
,而OH的增加
和SO 4 -
减小,从而导致系统的降低的氧化能力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号