当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantification of Active Apohemoglobin Heme-Binding Sites via Dicyanohemin Incorporation
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.biochem.7b00683 Ivan S. Pires 1 , Donald A. Belcher 1 , Andre F. Palmer 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.biochem.7b00683 Ivan S. Pires 1 , Donald A. Belcher 1 , Andre F. Palmer 1
Affiliation
|
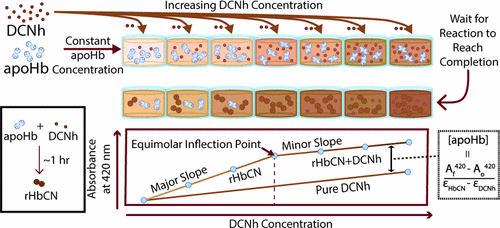
Apohemoglobin (apoHb) is produced by removing heme from hemoglobin (Hb). However, preparations of apoHb may contain damaged globins, which render total protein assays inaccurate for active apoHb quantification. Fortunately, apoHb heme-binding sites react with heme via the proximal histidine-F8 (His-F8) residue, which can be monitored spectrophotometrically. The bond between the His-F8 residue of apoHb and heme is vital for maintenance of fully functional and cooperative Hb. Additionally, most apoHb drug delivery applications facilitate hydrophobic drug incorporation inside the apoHb hydrophobic heme-binding pocket in which the His-F8 residue resides. This makes the His-F8 residue a proper target for apoHb activity quantification. In this work, dicyanohemin (DCNh), a stable monomeric porphyrin species, was used as a probe molecule to quantify active apoHb through monocyanohemin–His-F8 bond formation. ApoHb activity was quantified via the analysis of the 420 nm equilibrium absorbance of DCNh and apoHb mixtures. His-F8 saturation was determined by the presence of an inflection point from a plot of the 420 nm absorbance of a fixed concentration of apoHb against an increasing DCNh concentration. Various concentrations of a stock apoHb solution were tested to demonstrate the precision of the assay. The accuracy of the assay was assessed via spectral deconvolution, confirming His-F8 saturation at the inflection point. The effect of the heme-binding protein bovine serum albumin and precipitated apoHb on assay sensitivity was not significant. An analysis of the biophysical properties of reconstituted Hb confirmed heme-binding pocket activity. Taken together, this assay provides a simple and reliable method for determination of apoHb activity.
中文翻译:

通过双氰基血红素掺入的活性载铁血红蛋白血红素结合位点的定量
血红蛋白(apoHb)是通过从血红蛋白(Hb)中去除血红素而产生的。但是,载脂蛋白Hb的制剂中可能含有受损的球蛋白,这使得总蛋白检测对于载脂蛋白Hb的定量分析不准确。幸运的是,apoHb血红素结合位点通过近端的组氨酸-F8(His-F8)残基与血红素反应,可以通过分光光度法对其进行监测。apoHb的His-F8残基与血红素之间的键对于维持功能完整的Hb至关重要。另外,大多数apoHb药物递送应用促进疏水性药物掺入His-F8残基所在的apoHb疏水血红素结合袋内。这使His-F8残基成为apoHb活性定量的合适靶标。在这项工作中,一种稳定的卟啉单体双氰基合血红素(DCNh)用作探针分子,通过单氰基血红素-His-F8键形成来定量活性apoHb。通过分析DCNh和apoHb混合物的420 nm平衡吸光度来定量ApoHb活性。His-F8饱和度是根据固定浓度apoHb相对于DCNh浓度增加的420 nm吸光度图的拐点而确定的。测试了各种浓度的apoHb储备溶液,以证明测定的准确性。通过光谱解卷积评估测定的准确性,从而确定拐点处的His-F8饱和度。血红素结合蛋白牛血清白蛋白和沉淀的apoHb对测定灵敏度的影响不显着。重组血红蛋白的生物物理特性分析证实了血红素结合口袋活动。
更新日期:2017-09-20
中文翻译:

通过双氰基血红素掺入的活性载铁血红蛋白血红素结合位点的定量
血红蛋白(apoHb)是通过从血红蛋白(Hb)中去除血红素而产生的。但是,载脂蛋白Hb的制剂中可能含有受损的球蛋白,这使得总蛋白检测对于载脂蛋白Hb的定量分析不准确。幸运的是,apoHb血红素结合位点通过近端的组氨酸-F8(His-F8)残基与血红素反应,可以通过分光光度法对其进行监测。apoHb的His-F8残基与血红素之间的键对于维持功能完整的Hb至关重要。另外,大多数apoHb药物递送应用促进疏水性药物掺入His-F8残基所在的apoHb疏水血红素结合袋内。这使His-F8残基成为apoHb活性定量的合适靶标。在这项工作中,一种稳定的卟啉单体双氰基合血红素(DCNh)用作探针分子,通过单氰基血红素-His-F8键形成来定量活性apoHb。通过分析DCNh和apoHb混合物的420 nm平衡吸光度来定量ApoHb活性。His-F8饱和度是根据固定浓度apoHb相对于DCNh浓度增加的420 nm吸光度图的拐点而确定的。测试了各种浓度的apoHb储备溶液,以证明测定的准确性。通过光谱解卷积评估测定的准确性,从而确定拐点处的His-F8饱和度。血红素结合蛋白牛血清白蛋白和沉淀的apoHb对测定灵敏度的影响不显着。重组血红蛋白的生物物理特性分析证实了血红素结合口袋活动。



























 京公网安备 11010802027423号
京公网安备 11010802027423号