当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemistry and Spectroelectrochemistry of the Pu (III/IV) and (IV/VI) Couples in Nitric Acid Systems
Electroanalysis ( IF 3 ) Pub Date : 2017-09-20 , DOI: 10.1002/elan.201700465 Amanda M. Lines 1 , Susan R. Adami 1 , Amanda J. Casella 1 , Sergey I. Sinkov 1 , Gregg J. Lumetta 1 , Samuel A. Bryan 1
Electroanalysis ( IF 3 ) Pub Date : 2017-09-20 , DOI: 10.1002/elan.201700465 Amanda M. Lines 1 , Susan R. Adami 1 , Amanda J. Casella 1 , Sergey I. Sinkov 1 , Gregg J. Lumetta 1 , Samuel A. Bryan 1
Affiliation
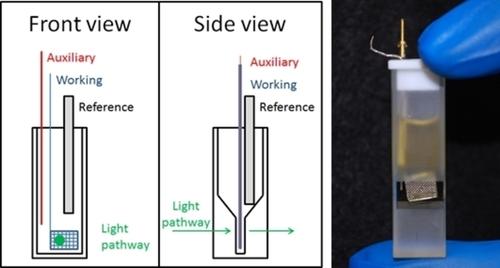
|
The solution chemistry of Pu in nitric acid is explored via electrochemistry and spectroelectrochemistry. By utilizing and comparing these techniques, an improved understanding of Pu behavior and its dependence on nitric acid concentration can be achieved. Here the Pu (III/IV) couple is characterized using cyclic voltammetry, square wave voltammetry, and a spectroelectrochemical Nernst step. Results indicate the formal reduction potential of the couple shifts negative with increasing acid concentration and reversible electrochemistry is no longer attainable above 6 M HNO3. Spectroelectrochemistry is also used to explore the irreversible oxidation of Pu(IV) to Pu(VI) and shine light on the mechanism and acid dependence of the redox reaction.
中文翻译:

硝酸系统中 Pu (III/IV) 和 (IV/VI) 对的电化学和光谱电化学
通过电化学和光谱电化学探索了 Pu 在硝酸中的溶液化学。通过利用和比较这些技术,可以更好地了解 Pu 行为及其对硝酸浓度的依赖性。在这里,Pu (III/IV) 对使用循环伏安法、方波伏安法和光谱电化学能斯特步骤进行表征。结果表明,随着酸浓度的增加,这对偶的形式还原电位变为负值,并且在 6 M HNO3 以上不再获得可逆电化学。光谱电化学还用于探索 Pu(IV) 到 Pu(VI) 的不可逆氧化,并揭示氧化还原反应的机制和酸依赖性。
更新日期:2017-09-20
中文翻译:

硝酸系统中 Pu (III/IV) 和 (IV/VI) 对的电化学和光谱电化学
通过电化学和光谱电化学探索了 Pu 在硝酸中的溶液化学。通过利用和比较这些技术,可以更好地了解 Pu 行为及其对硝酸浓度的依赖性。在这里,Pu (III/IV) 对使用循环伏安法、方波伏安法和光谱电化学能斯特步骤进行表征。结果表明,随着酸浓度的增加,这对偶的形式还原电位变为负值,并且在 6 M HNO3 以上不再获得可逆电化学。光谱电化学还用于探索 Pu(IV) 到 Pu(VI) 的不可逆氧化,并揭示氧化还原反应的机制和酸依赖性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号