Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic and Thermodynamic Control in Porphyrin and Phthalocyanine Self-Assembled Monolayers
Langmuir ( IF 3.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.langmuir.7b02672 K. W. Hipps 1 , Ursula Mazur 1
Langmuir ( IF 3.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.langmuir.7b02672 K. W. Hipps 1 , Ursula Mazur 1
Affiliation
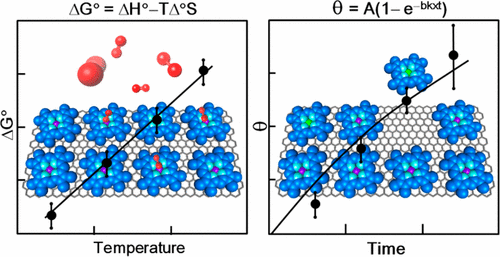
|
Porphyrins and phthalocyanines are ubiquitous in modern science and technology. Their stability, redox properties, and photoresponse make them candidates for numerous applications. Many of these applications rely on thin films, and these are critically dependent on the first monolayer. In this article, we focus on noncovalently bound self-assembled monolayers of porphyrins and phthalocyanines at the solution–solid interface with special emphasis on the kinetic and thermodynamic processes that define the films and their reaction chemistry. We first discuss the difference between film-formation kinetics and desorption kinetics from fully formed films. We then present evidence that many of these monolayers are controlled by adsorption kinetics and are not in thermodynamic equilibrium. Measurement of the solution–solid interface desorption energy by scanning tunneling microscopy is discussed, and data is presented for cobalt, nickel, and free base octaethylporphyrin. The activation energy for the desorption of these compounds into phenyloctane is about half of the computed desorption energy in vacuum, and this is discussed in terms of the role of the solvent. Preexponential factors are very low compared to desorption into vacuum, and this is attributed to a reduction in the entropy of activation due to the participation of solvent in the transition state. An example of the use of relative desorption kinetics to create a new binary surface structure is given. It is suggested that this is a synthesis route that may have been missed because of the large difference in solution concentrations required to drive binary film formation. Attention then turns to the axial reaction chemistry of metalloporphyrins and metallophthalocyanines supported on conducting surfaces. We show several examples of chemistry unique to the supported complexes: cases where the metal binds ligands more readily and cases where the substrate induces ligand loss. Understanding this new axial coordination chemistry is of great importance in catalysis, sensing, and the growth of 3D materials from a self-assembled template.
中文翻译:

卟啉和酞菁自组装单分子膜的动力学和热力学控制
卟啉和酞菁在现代科学技术中无处不在。它们的稳定性,氧化还原特性和光响应性使其成为众多应用的候选者。这些应用中的许多都依赖于薄膜,并且这些薄膜严重依赖于第一单层。在本文中,我们将重点研究溶液-固体界面上卟啉和酞菁的非共价结合自组装单分子膜,并特别强调定义膜及其反应化学的动力学和热力学过程。我们首先讨论成膜动力学与完全成型膜的解吸动力学之间的差异。然后,我们提供证据表明,许多这些单分子层均受吸附动力学控制,并且不处于热力学平衡状态。讨论了通过扫描隧道显微镜对溶液-固体界面脱附能的测量,并给出了钴,镍和游离碱八乙基卟啉的数据。这些化合物解吸为苯辛烷的活化能约为真空中计算出的解吸能的一半,这将根据溶剂的作用进行讨论。与解吸到真空中相比,预指数因子非常低,这归因于由于溶剂参与过渡态而引起的活化熵的降低。给出了使用相对解吸动力学产生新的二元表面结构的例子。建议这是一条合成路线,由于驱动二元膜形成所需的溶液浓度差异很大,因此可能会错过该合成路线。然后将注意力转向负载在导电表面上的金属卟啉和金属酞菁的轴向反应化学。我们展示了几种对所支持的配合物而言独特的化学反应的例子:金属更容易与配体结合的情况以及底物诱导配体损失的情况。了解这种新的轴向配位化学对于从自组装模板催化,感测和3D材料的生长非常重要。
更新日期:2017-09-20
中文翻译:

卟啉和酞菁自组装单分子膜的动力学和热力学控制
卟啉和酞菁在现代科学技术中无处不在。它们的稳定性,氧化还原特性和光响应性使其成为众多应用的候选者。这些应用中的许多都依赖于薄膜,并且这些薄膜严重依赖于第一单层。在本文中,我们将重点研究溶液-固体界面上卟啉和酞菁的非共价结合自组装单分子膜,并特别强调定义膜及其反应化学的动力学和热力学过程。我们首先讨论成膜动力学与完全成型膜的解吸动力学之间的差异。然后,我们提供证据表明,许多这些单分子层均受吸附动力学控制,并且不处于热力学平衡状态。讨论了通过扫描隧道显微镜对溶液-固体界面脱附能的测量,并给出了钴,镍和游离碱八乙基卟啉的数据。这些化合物解吸为苯辛烷的活化能约为真空中计算出的解吸能的一半,这将根据溶剂的作用进行讨论。与解吸到真空中相比,预指数因子非常低,这归因于由于溶剂参与过渡态而引起的活化熵的降低。给出了使用相对解吸动力学产生新的二元表面结构的例子。建议这是一条合成路线,由于驱动二元膜形成所需的溶液浓度差异很大,因此可能会错过该合成路线。然后将注意力转向负载在导电表面上的金属卟啉和金属酞菁的轴向反应化学。我们展示了几种对所支持的配合物而言独特的化学反应的例子:金属更容易与配体结合的情况以及底物诱导配体损失的情况。了解这种新的轴向配位化学对于从自组装模板催化,感测和3D材料的生长非常重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号