当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Does the Residues Chirality Modify the Conformation of a Cyclo-Dipeptide? Vibrational Spectroscopy of Protonated Cyclo-diphenylalanine in the Gas Phase
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpca.7b06159 Ivan Alata 1 , Ariel Pérez-Mellor 1 , Feriel Ben Nasr 1, 2 , Debora Scuderi 3 , Vincent Steinmetz 3 , Fabrice Gobert 3 , Nejm-Eddine Jaïdane 2 , Anne Zehnacker-Rentien 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jpca.7b06159 Ivan Alata 1 , Ariel Pérez-Mellor 1 , Feriel Ben Nasr 1, 2 , Debora Scuderi 3 , Vincent Steinmetz 3 , Fabrice Gobert 3 , Nejm-Eddine Jaïdane 2 , Anne Zehnacker-Rentien 1
Affiliation
|
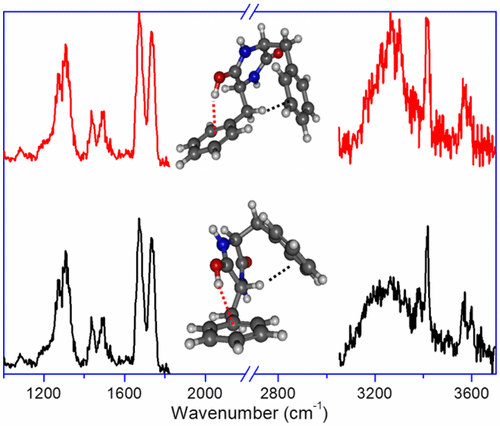
The structure of a protonated diketopiperazine dipeptide, cyclo-diphenylalanine, is studied by means of infrared multiple photon dissociation spectroscopy combined with quantum chemical calculations. Protonation exclusively occurs on the oxygen site and, in the most stable conformer, results to an intramolecular OH···π interaction, accompanied by a CH···π interaction. Higher-energy conformers with free OH and NH···π interactions are observed as well, due to kinetic trapping. Optimization of the intramolecular interactions involving the aromatic ring dictates the geometry of the benzyl substituents. Changing the chirality of one of the residues has consequences on the CH···π interaction, which is of CαH···π nature for LD, while LL shows a CβH···π interaction. Higher-energy conformers also display some differences in the nature of the intramolecular interactions.
中文翻译:

残基手性是否会修饰环二肽的构象?气相中质子化的环二苯丙氨酸的振动光谱
通过红外多光子解离光谱结合量子化学计算研究了质子化的二酮哌嗪二肽环二苯丙氨酸的结构。质子化仅在氧位点发生,并且在最稳定的构象异构体中,导致分子内OH···π相互作用,并伴有CH···π相互作用。由于动力学陷阱,还观察到具有自由的OH和NH···π相互作用的高能构象体。涉及芳环的分子内相互作用的优化决定了苄基取代基的几何形状。改变的残基中的一个的手性对CH···π相互作用,这是C的后果α ħ···π性质为LD,而LL示出一个C βH···π相互作用。高能构象物在分子内相互作用的性质上也表现出一些差异。
更新日期:2017-09-20
中文翻译:

残基手性是否会修饰环二肽的构象?气相中质子化的环二苯丙氨酸的振动光谱
通过红外多光子解离光谱结合量子化学计算研究了质子化的二酮哌嗪二肽环二苯丙氨酸的结构。质子化仅在氧位点发生,并且在最稳定的构象异构体中,导致分子内OH···π相互作用,并伴有CH···π相互作用。由于动力学陷阱,还观察到具有自由的OH和NH···π相互作用的高能构象体。涉及芳环的分子内相互作用的优化决定了苄基取代基的几何形状。改变的残基中的一个的手性对CH···π相互作用,这是C的后果α ħ···π性质为LD,而LL示出一个C βH···π相互作用。高能构象物在分子内相互作用的性质上也表现出一些差异。


























 京公网安备 11010802027423号
京公网安备 11010802027423号