当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Bis-benzopyrroloisoquinoline Alkaloid Incorporating a Cyclobutane Core and a Chlorophenanthroindolizidine Alkaloid with Cytotoxic Activity from Ficus fistulosa var. tengerensis
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00500 Amjad Ayad Qatran Al-Khdhairawi 1 , Premanand Krishnan 1 , Chun-Wai Mai 2 , Felicia Fei-Lei Chung 3 , Chee-Onn Leong 2, 3 , Kien-Thai Yong 4 , Kam-Weng Chong 5 , Yun-Yee Low 5 , Toh-Seok Kam 5 , Kuan-Hon Lim 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00500 Amjad Ayad Qatran Al-Khdhairawi 1 , Premanand Krishnan 1 , Chun-Wai Mai 2 , Felicia Fei-Lei Chung 3 , Chee-Onn Leong 2, 3 , Kien-Thai Yong 4 , Kam-Weng Chong 5 , Yun-Yee Low 5 , Toh-Seok Kam 5 , Kuan-Hon Lim 1
Affiliation
|
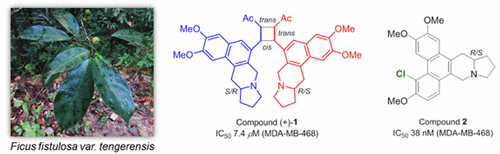
Tengerensine (1), isolated as a racemate and constituted from a pair of bis-benzopyrroloisoquinoline enantiomers, and tengechlorenine (2), purified as a scalemic mixture and constituted from a pair of chlorinated phenanthroindolizidine enantiomers, were isolated from the leaves of Ficus fistulosa var. tengerensis, along with three other known alkaloids. The structures of 1 and 2 were determined by spectroscopic data interpretation and X-ray diffraction analysis. The enantiomers of 1 were separated by chiral-phase HPLC, and the absolute configurations of (+)-1 and (−)-1 were established via experimental and calculated ECD data. Compound 1 is notable in being a rare unsymmetrical cyclobutane adduct and is the first example of a dimeric benzopyrroloisoquinoline alkaloid, while compound 2 represents the first naturally occurring halogenated phenanthroindolizidine alkaloid. Compound (+)-1 displayed a selective in vitro cytotoxic effect against MDA-MB-468 cells (IC50 7.4 μM), while compound 2 showed pronounced in vitro cytotoxic activity against all three breast cancer cell lines tested (MDA-MB-468, MDA-MB-231, and MCF7; IC50 values of 0.038–0.91 μM).
中文翻译:

结合有环丁烷核心和具有菲毒无花果变种的细胞毒活性的氯代菲咯啉吲哚并立生物碱的双-苯并吡咯并异喹啉生物碱。腾格里斯
Tengerensine(1),分离为外消旋体,并从一对双benzopyrroloisoquinoline对映异构体,和tengechlorenine(构成2),纯化的作为消旋混合物和从一对氯化菲骈吲哚对映体的构成中,从叶分离的无花果fistulosa变种。tengerensis和其他三种已知的生物碱。1和2的结构通过光谱数据解释和X射线衍射分析确定。通过手性相HPLC分离1的对映体,以及(+)- 1和(-)- 1的绝对构型通过实验和计算得出的ECD数据确定。化合物1值得注意的是稀有的不对称环丁烷加合物,并且是二聚苯并吡咯并异喹啉碱生物碱的第一个实例,而化合物2代表第一个天然存在的卤代菲咯啉吲哚生物碱。化合物(+)- 1对MDA-MB-468细胞(IC 50 7.4μM)表现出选择性的体外细胞毒性作用,而化合物2对所有测试的三种乳腺癌细胞系(MDA-MB-468 )表现出明显的体外细胞毒性活性,MDA-MB-231和MCF7; IC 50值为0.038-0.91μM)。
更新日期:2017-09-19
中文翻译:

结合有环丁烷核心和具有菲毒无花果变种的细胞毒活性的氯代菲咯啉吲哚并立生物碱的双-苯并吡咯并异喹啉生物碱。腾格里斯
Tengerensine(1),分离为外消旋体,并从一对双benzopyrroloisoquinoline对映异构体,和tengechlorenine(构成2),纯化的作为消旋混合物和从一对氯化菲骈吲哚对映体的构成中,从叶分离的无花果fistulosa变种。tengerensis和其他三种已知的生物碱。1和2的结构通过光谱数据解释和X射线衍射分析确定。通过手性相HPLC分离1的对映体,以及(+)- 1和(-)- 1的绝对构型通过实验和计算得出的ECD数据确定。化合物1值得注意的是稀有的不对称环丁烷加合物,并且是二聚苯并吡咯并异喹啉碱生物碱的第一个实例,而化合物2代表第一个天然存在的卤代菲咯啉吲哚生物碱。化合物(+)- 1对MDA-MB-468细胞(IC 50 7.4μM)表现出选择性的体外细胞毒性作用,而化合物2对所有测试的三种乳腺癌细胞系(MDA-MB-468 )表现出明显的体外细胞毒性活性,MDA-MB-231和MCF7; IC 50值为0.038-0.91μM)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号