当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular basis for the unusual ring reconstruction in fungal meroterpenoid biogenesis
Nature Chemical Biology ( IF 14.8 ) Pub Date : 2017-07-31 00:00:00 , DOI: 10.1038/nchembio.2443 Takahiro Mori , Taiki Iwabuchi , Shotaro Hoshino , Hang Wang , Yudai Matsuda , Ikuro Abe
Nature Chemical Biology ( IF 14.8 ) Pub Date : 2017-07-31 00:00:00 , DOI: 10.1038/nchembio.2443 Takahiro Mori , Taiki Iwabuchi , Shotaro Hoshino , Hang Wang , Yudai Matsuda , Ikuro Abe
|
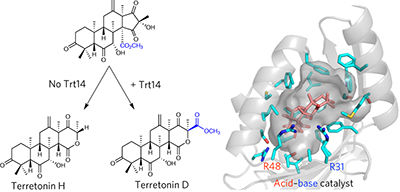
Trt14 from Aspergillus terreus is involved in unusual skeletal reconstruction during the biosynthesis of the fungal meroterpenoid terretonin. Detailed in vitro characterization revealed that this novel multifunctional enzyme catalyzes not only the D-ring expansion via intramolecular methoxy rearrangement, but also the hydrolysis of the expanded D-ring. The X-ray crystal structures of Trt14, in complex with substrate or product, and two Trt14 homologs, AusH and PrhC from Aspergillus nidulans and Penicillium brasilianum, respectively, indicated similar overall structures to those of the NTF2-like superfamily of enzymes, despite lacking sequence and functional similarities. Moreover, we gained structural insight into the mechanism of the Trt14-catalyzed ring reconstruction from the in-crystal enzyme reaction and site-directed mutagenesis to show that this reaction involves sequential ester bond cleavage and formation. Structural comparison of Trt14 and its homologs suggests that the enzymes in this new superfamily employ similar acid–base chemistry to diversify the molecular architecture of fungal meroterpenoids.
中文翻译:

真菌类萜生物发生中异常环重建的分子基础
土生曲霉的Trt14参与了真菌类戊二烯萜素的生物合成过程中异常的骨骼重建。详细的体外表征表明,这种新型多功能酶不仅通过分子内甲氧基重排催化D环的扩展,而且还催化扩展的D环的水解。Trt14的X射线晶体结构,与底物或产物形成复合物,以及构巢曲霉和巴西青霉的两个Trt14同源物AusH和PrhC,尽管缺乏序列和功能相似性,但分别表示与酶的NTF2样超家族相似的总体结构。此外,我们从晶体内酶反应和定点诱变获得了对Trt14催化的环重构机理的结构见解,以表明该反应涉及顺序的酯键裂解和形成。Trt14及其同源物的结构比较表明,这个新的超家族中的酶采用相似的酸碱化学方法来使真菌类胡萝卜素的分子结构多样化。
更新日期:2017-09-20
中文翻译:

真菌类萜生物发生中异常环重建的分子基础
土生曲霉的Trt14参与了真菌类戊二烯萜素的生物合成过程中异常的骨骼重建。详细的体外表征表明,这种新型多功能酶不仅通过分子内甲氧基重排催化D环的扩展,而且还催化扩展的D环的水解。Trt14的X射线晶体结构,与底物或产物形成复合物,以及构巢曲霉和巴西青霉的两个Trt14同源物AusH和PrhC,尽管缺乏序列和功能相似性,但分别表示与酶的NTF2样超家族相似的总体结构。此外,我们从晶体内酶反应和定点诱变获得了对Trt14催化的环重构机理的结构见解,以表明该反应涉及顺序的酯键裂解和形成。Trt14及其同源物的结构比较表明,这个新的超家族中的酶采用相似的酸碱化学方法来使真菌类胡萝卜素的分子结构多样化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号