当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2
Nature Chemical Biology ( IF 14.8 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1038/nchembio.2470 Bastien Bissaro , Åsmund K Røhr , Gerdt Müller , Piotr Chylenski , Morten Skaugen , Zarah Forsberg , Svein J Horn , Gustav Vaaje-Kolstad , Vincent G H Eijsink
Nature Chemical Biology ( IF 14.8 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1038/nchembio.2470 Bastien Bissaro , Åsmund K Røhr , Gerdt Müller , Piotr Chylenski , Morten Skaugen , Zarah Forsberg , Svein J Horn , Gustav Vaaje-Kolstad , Vincent G H Eijsink
|
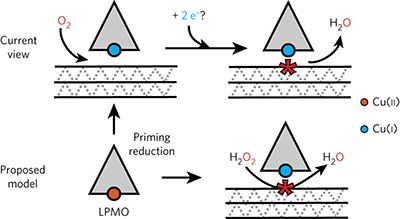
Enzymes currently known as lytic polysaccharide monooxygenases (LPMOs) play an important role in the conversion of recalcitrant polysaccharides, but their mode of action has remained largely enigmatic. It is generally believed that catalysis by LPMOs requires molecular oxygen and a reductant that delivers two electrons per catalytic cycle. Using enzyme assays, mass spectrometry and experiments with labeled oxygen atoms, we show here that H2O2, rather than O2, is the preferred co-substrate of LPMOs. By controlling H2O2 supply, stable reaction kinetics are achieved, the LPMOs work in the absence of O2, and the reductant is consumed in priming rather than in stoichiometric amounts. The use of H2O2 by a monocopper enzyme that is otherwise cofactor-free offers new perspectives regarding the mode of action of copper enzymes. Furthermore, these findings have implications for the enzymatic conversion of biomass in Nature and in industrial biorefining.
中文翻译:

铜酶对多糖的氧化裂解取决于H2O2
目前被称为溶解性多糖单加氧酶(LPMO)的酶在顽固性多糖的转化中起着重要的作用,但是它们的作用方式在很大程度上一直是令人困惑的。通常认为,LPMO的催化需要分子氧和在每个催化循环中传递两个电子的还原剂。使用酶分析,质谱和标记氧原子的实验,我们在这里表明H 2 O 2而不是O 2是LPMO的首选共底物。通过控制H 2 O 2的供应,可获得稳定的反应动力学,LPMO在没有O 2的情况下起作用,还原剂消耗的是引发剂,而不是化学计量的量。不含铜的辅酶的单铜酶对H 2 O 2的使用为铜酶的作用方式提供了新的见解。此外,这些发现对自然界和工业生物精制过程中生物质的酶转化具有重要意义。
更新日期:2017-09-20
中文翻译:

铜酶对多糖的氧化裂解取决于H2O2
目前被称为溶解性多糖单加氧酶(LPMO)的酶在顽固性多糖的转化中起着重要的作用,但是它们的作用方式在很大程度上一直是令人困惑的。通常认为,LPMO的催化需要分子氧和在每个催化循环中传递两个电子的还原剂。使用酶分析,质谱和标记氧原子的实验,我们在这里表明H 2 O 2而不是O 2是LPMO的首选共底物。通过控制H 2 O 2的供应,可获得稳定的反应动力学,LPMO在没有O 2的情况下起作用,还原剂消耗的是引发剂,而不是化学计量的量。不含铜的辅酶的单铜酶对H 2 O 2的使用为铜酶的作用方式提供了新的见解。此外,这些发现对自然界和工业生物精制过程中生物质的酶转化具有重要意义。

























 京公网安备 11010802027423号
京公网安备 11010802027423号