当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The impact of ionic liquids on the coordination of anions with solvatochromic copper complexes
Dalton Transactions ( IF 4 ) Pub Date : 2017-08-23 00:00:00 , DOI: 10.1039/c7dt02372c O. Kuzmina 1, 2, 3, 4 , N. H. Hassan 1, 2, 3, 4, 5 , L. Patel 1, 2, 3, 4 , C. Ashworth 1, 2, 3, 4 , E. Bakis 1, 2, 3, 4 , A. J. P. White 1, 2, 3, 4 , P. A. Hunt 1, 2, 3, 4 , T. Welton 1, 2, 3, 4
Dalton Transactions ( IF 4 ) Pub Date : 2017-08-23 00:00:00 , DOI: 10.1039/c7dt02372c O. Kuzmina 1, 2, 3, 4 , N. H. Hassan 1, 2, 3, 4, 5 , L. Patel 1, 2, 3, 4 , C. Ashworth 1, 2, 3, 4 , E. Bakis 1, 2, 3, 4 , A. J. P. White 1, 2, 3, 4 , P. A. Hunt 1, 2, 3, 4 , T. Welton 1, 2, 3, 4
Affiliation
|
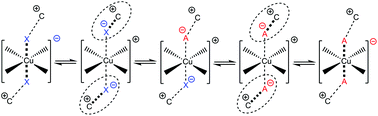
Solvatochromic transition metal (TM)-complexes with weakly associating counter-anions are often used to evaluate traditional neutral solvent and anion coordination ability. However, when employed in ionic liquids (IL) many of the common assumptions made are no longer reliable. This study investigates the coordinating ability of weakly coordinating IL anions in traditional solvents and within IL solvents employing a range of solvatochromic copper complexes. Complexes of the form [Cu(acac)(tmen)][X] (acac = acetylacetonate, tmen = tetramethylethylenediamine) where [X]− = [ClO4]−, Cl−, [NO3]−, [SCN]−, [OTf]−, [NTf2]− and [PF6]− have been synthesised and characterised both experimentally and computationally. ILs based on these anions and imidazolium and pyrrolidinium cations, some of which are functionalised with hydroxyl and nitrile groups, have been examined. IL-anion coordination has been investigated and compared to typical weakly coordinating anions. We have found there is potential for competition at the Cu-centre and cases of anions traditionally assigned as weakly associating that demonstrate a stronger than expected level of coordinating ability within ILs. [Cu(acac)(tmen)][PF6] is shown to contain the least coordinating anion and is established as the most sensitive probe studied here. Using this probe, the donor numbers (DNs) of ILs have been determined. Relative donor ability is further confirmed based on the UV-Vis of a neutral complex, [Cu(sacsac)2] (sacsac = dithioacetylacetone), and DNs evaluated via23Na NMR spectroscopy. We demonstrate that ILs can span a wide donor range, similar in breadth to conventional solvents.
中文翻译:

离子液体对阴离子与溶剂致变色铜配合物配位的影响
具有弱缔合抗衡阴离子的溶剂变色过渡金属(TM)络合物通常用于评估传统的中性溶剂和阴离子的配位能力。但是,在离子液体(IL)中使用时,许多常见的假设不再可靠。这项研究研究了在弱溶剂中,IL阴离子在传统溶剂中以及在使用一系列溶剂变色铜络合物的IL溶剂中的弱配位能力。[Cu(acac)(tmen)] [X](acac =乙酰丙酮酸酯,tmen =四甲基乙二胺)形式的络合物,其中[X] - = [ClO 4 ] -,Cl -,[NO 3 ] -,[SCN] -,[OTf] −,[NTf 2已经合成了] -和[PF 6 ] -并在实验和计算上进行了表征。已经检查了基于这些阴离子以及咪唑鎓和吡咯烷鎓阳离子的IL,其中一些被羟基和腈基官能化。已对IL-阴离子配位进行了研究,并将其与典型的弱配位阴离子进行了比较。我们发现,在铜中心存在竞争的潜力,传统上被认为是弱缔合的阴离子的情况表明,离子液体中的协调能力强于预期水平。[Cu(acac)(tmen)] [PF 6所示的]含有最少的配位阴离子,并被确定为此处研究的最敏感的探针。使用该探针,已经确定了IL的供体数目(DN)。基于中性配合物[Cu(sacsac)2 ](sacsac =二硫代乙酰丙酮)的UV-Vis和通过23 Na NMR光谱评估的DN,进一步确定了相对供体的能力。我们证明ILs可以跨越较宽的供体范围,其宽度类似于常规溶剂。
更新日期:2017-09-20
中文翻译:

离子液体对阴离子与溶剂致变色铜配合物配位的影响
具有弱缔合抗衡阴离子的溶剂变色过渡金属(TM)络合物通常用于评估传统的中性溶剂和阴离子的配位能力。但是,在离子液体(IL)中使用时,许多常见的假设不再可靠。这项研究研究了在弱溶剂中,IL阴离子在传统溶剂中以及在使用一系列溶剂变色铜络合物的IL溶剂中的弱配位能力。[Cu(acac)(tmen)] [X](acac =乙酰丙酮酸酯,tmen =四甲基乙二胺)形式的络合物,其中[X] - = [ClO 4 ] -,Cl -,[NO 3 ] -,[SCN] -,[OTf] −,[NTf 2已经合成了] -和[PF 6 ] -并在实验和计算上进行了表征。已经检查了基于这些阴离子以及咪唑鎓和吡咯烷鎓阳离子的IL,其中一些被羟基和腈基官能化。已对IL-阴离子配位进行了研究,并将其与典型的弱配位阴离子进行了比较。我们发现,在铜中心存在竞争的潜力,传统上被认为是弱缔合的阴离子的情况表明,离子液体中的协调能力强于预期水平。[Cu(acac)(tmen)] [PF 6所示的]含有最少的配位阴离子,并被确定为此处研究的最敏感的探针。使用该探针,已经确定了IL的供体数目(DN)。基于中性配合物[Cu(sacsac)2 ](sacsac =二硫代乙酰丙酮)的UV-Vis和通过23 Na NMR光谱评估的DN,进一步确定了相对供体的能力。我们证明ILs可以跨越较宽的供体范围,其宽度类似于常规溶剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号