当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-free oxidative cyclization of 2-aminobenzothiazoles and cyclic ketones enabled by the combination of elemental sulfur and oxygen
Green Chemistry ( IF 9.8 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7gc02014g Yanjun Xie 1, 2, 3, 4, 5 , Xiangui Chen 1, 2, 3, 4, 5 , Zhen Wang 1, 2, 3, 4, 5 , Huawen Huang 1, 2, 3, 4, 5 , Bing Yi 5, 6, 7, 8 , Guo-Jun Deng 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7gc02014g Yanjun Xie 1, 2, 3, 4, 5 , Xiangui Chen 1, 2, 3, 4, 5 , Zhen Wang 1, 2, 3, 4, 5 , Huawen Huang 1, 2, 3, 4, 5 , Bing Yi 5, 6, 7, 8 , Guo-Jun Deng 1, 2, 3, 4, 5
Affiliation
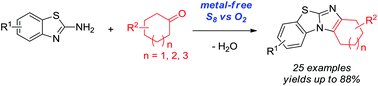
|
Metal-free oxidative cyclization for the one-pot synthesis of benzo[d]imidazo[2,1-b]thiazoles from 2-aminobenzothiazoles and cyclic ketones is described. Elemental sulfur combined with molecular oxygen as the benign co-oxidant was found to be unique and highly effective to promote this transformation without the aid of any metal salts. Various cyclic ketones smoothly reacted with 2-aminobenzothiazoles to give functional benzo[d]imidazo[2,1-b]thiazoles in good to very high yields, which thereby demonstrated the synthetic convergence of this methodology.
中文翻译:

通过元素硫和氧的结合实现2-氨基苯并噻唑和环状酮的无金属氧化环化
描述了用于从2-氨基苯并噻唑和环状酮一锅合成苯并[ d ]咪唑并[ 2,1- b ]噻唑的无金属氧化环化反应。发现元素硫与分子氧结合作为良性助氧化剂是独特的,并且高度有效地促进了这种转变,而无需任何金属盐的帮助。各种环状酮与2-氨基苯并噻唑平稳反应,以高至非常高的收率得到官能化的苯并[ d ]咪唑并[ 2,1- b ]噻唑,从而证明了该方法的合成收敛性。
更新日期:2017-09-19
中文翻译:

通过元素硫和氧的结合实现2-氨基苯并噻唑和环状酮的无金属氧化环化
描述了用于从2-氨基苯并噻唑和环状酮一锅合成苯并[ d ]咪唑并[ 2,1- b ]噻唑的无金属氧化环化反应。发现元素硫与分子氧结合作为良性助氧化剂是独特的,并且高度有效地促进了这种转变,而无需任何金属盐的帮助。各种环状酮与2-氨基苯并噻唑平稳反应,以高至非常高的收率得到官能化的苯并[ d ]咪唑并[ 2,1- b ]噻唑,从而证明了该方法的合成收敛性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号