当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoorganocatalytic synthesis of lactones via a selective C–H activation–alkylation of alcohols
Green Chemistry ( IF 9.8 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7gc01903c Nikolaos Kaplaneris 1, 2, 3, 4, 5 , Aikaterini Bisticha 1, 2, 3, 4, 5 , Giorgos N. Papadopoulos 1, 2, 3, 4, 5 , Dimitris Limnios 1, 2, 3, 4, 5 , Christoforos G. Kokotos 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7gc01903c Nikolaos Kaplaneris 1, 2, 3, 4, 5 , Aikaterini Bisticha 1, 2, 3, 4, 5 , Giorgos N. Papadopoulos 1, 2, 3, 4, 5 , Dimitris Limnios 1, 2, 3, 4, 5 , Christoforos G. Kokotos 1, 2, 3, 4, 5
Affiliation
|
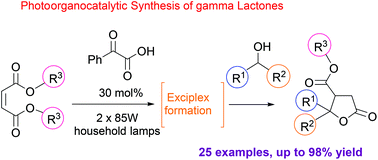
Selective C–H activation is an area of growing importance in modern organic chemistry. Herein, we report our efforts in combining organocatalysis and photocatalysis for the development of a highly efficient and selective visible-light mediated protocol for the C–H activation and addition of various alcohols to a plethora of Michael acceptors, followed by a cyclization reaction leading to lactones, a repeatedly occurring motif in nature. Utilizing phenylglyoxylic acid as the photocatalyst and common household bulbs as the light source, we describe a versatile α-alkylation/lactonization of alcohols with α,β-unsaturated esters leading to products in excellent yields. The reaction mechanism was extensively studied.
中文翻译:

通过醇的选择性C–H活化–烷基化反应进行光有机催化内酯的合成
选择性C–H活化是现代有机化学中日益重要的一个领域。在此,我们报告了我们在有机催化和光催化相结合方面的努力,以开发高效且选择性的可见光介导的C–H活化方案,并向大量Michael受体添加各种醇,然后进行环化反应,内酯,自然界中反复出现的基序。我们使用苯乙醛酸作为光催化剂,并用普通的家用灯泡作为光源,我们描述了醇与α,β-不饱和酯的多用途α-烷基化/内酯化,可产生高收率的产品。反应机理得到了广泛的研究。
更新日期:2017-09-19
中文翻译:

通过醇的选择性C–H活化–烷基化反应进行光有机催化内酯的合成
选择性C–H活化是现代有机化学中日益重要的一个领域。在此,我们报告了我们在有机催化和光催化相结合方面的努力,以开发高效且选择性的可见光介导的C–H活化方案,并向大量Michael受体添加各种醇,然后进行环化反应,内酯,自然界中反复出现的基序。我们使用苯乙醛酸作为光催化剂,并用普通的家用灯泡作为光源,我们描述了醇与α,β-不饱和酯的多用途α-烷基化/内酯化,可产生高收率的产品。反应机理得到了广泛的研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号