当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isomerization of glucose to fructose catalyzed by lithium bromide in water
Green Chemistry ( IF 9.8 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1039/c7gc02145c Chang Geun Yoo 1, 2, 3, 4 , Ning Li 1, 2, 3, 4 , Melanie Swannell 1, 2, 3, 4 , Xuejun Pan 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1039/c7gc02145c Chang Geun Yoo 1, 2, 3, 4 , Ning Li 1, 2, 3, 4 , Melanie Swannell 1, 2, 3, 4 , Xuejun Pan 1, 2, 3, 4
Affiliation
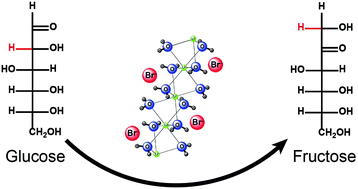
|
This study demonstrated that glucose could be isomerized to fructose in the concentrated aqueous solution of lithium bromide (LiBr) under mild conditions. The isomerization mechanisms were studied via isotopic labeling experiments. It was found that both the cation (Li+) and the anion (Br−) in the system catalyzed the isomerization of glucose to fructose. Br− catalyzed the isomerization through the proton transfer mechanism via an enediol intermediate, while Li+ did it through an intramolecular hydride shift mechanism from C2 to C1. The estimation using quantitative 13C-NMR analysis indicated that Br− catalyzed approximately 85% of the isomerization, while Li+ was responsible for the remaining 15%. The results indicated that 31% of the fructose was produced from glucose in LiBr trihydrate at 120 °C for 15 min. The outcomes of this study provided not only a better understanding of and insights into the sugar transformations in the LiBr solution but also an alternative approach to produce fructose from glucose.
中文翻译:

水中溴化锂催化葡萄糖异构化为果糖
这项研究表明,在温和的条件下,葡萄糖可以在浓缩的溴化锂(LiBr)水溶液中异构化为果糖。通过同位素标记实验研究了异构化机理。结果发现,无论是阳离子(李+),阴离子(BR - )系统催化葡萄糖异构化为果糖。BR -催化的通过质子转移机构异构化通过烯二醇中间体,而锂+做到了通过分子内氢化换档机构从C2至C1。使用定量估计13 C-NMR分析表明,溴-Li +催化了约85%的异构化反应,而Li +负责了其余15%的反应。结果表明,果糖中的31%是由葡萄糖在三水合溴化锂中于120°C持续15分钟产生的。这项研究的结果不仅提供了对LiBr溶液中糖转化的更好理解和深入了解,而且还提供了一种从葡萄糖生产果糖的替代方法。
更新日期:2017-09-19
中文翻译:

水中溴化锂催化葡萄糖异构化为果糖
这项研究表明,在温和的条件下,葡萄糖可以在浓缩的溴化锂(LiBr)水溶液中异构化为果糖。通过同位素标记实验研究了异构化机理。结果发现,无论是阳离子(李+),阴离子(BR - )系统催化葡萄糖异构化为果糖。BR -催化的通过质子转移机构异构化通过烯二醇中间体,而锂+做到了通过分子内氢化换档机构从C2至C1。使用定量估计13 C-NMR分析表明,溴-Li +催化了约85%的异构化反应,而Li +负责了其余15%的反应。结果表明,果糖中的31%是由葡萄糖在三水合溴化锂中于120°C持续15分钟产生的。这项研究的结果不仅提供了对LiBr溶液中糖转化的更好理解和深入了解,而且还提供了一种从葡萄糖生产果糖的替代方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号