当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bone Marrow-Derived Tenascin-C Attenuates Cardiac Hypertrophy by Controlling Inflammation
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-09-01 , DOI: 10.1016/j.jacc.2017.07.789 Lei Song , Lai Wang , Fuqiang Li , Ada Yukht , Minghui Qin , Haley Ruther , Mingjie Yang , Aurelio Chaux , Prediman K. Shah , Behrooz G. Sharifi
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-09-01 , DOI: 10.1016/j.jacc.2017.07.789 Lei Song , Lai Wang , Fuqiang Li , Ada Yukht , Minghui Qin , Haley Ruther , Mingjie Yang , Aurelio Chaux , Prediman K. Shah , Behrooz G. Sharifi
|
BACKGROUND
Tenascin-C (TNC) is a highly conserved matricellular protein with a distinct expression pattern during development and disease. Remodeling of the left ventricle (LV) in response to pressure overload leads to the re-expression of the fetal gene program. OBJECTIVES
The aim of this study was to investigate the function of TNC in cardiac hypertrophy in response to pressure overload. METHODS
Pressure overload was induced in TNC knockout and wild-type mice by constricting their abdominal aorta or by infusion of angiotensin II. Echocardiography, immunostaining, flow cytometry, quantitative real-time polymerase chain reaction, and reciprocal bone marrow transplantation were used to evaluate the effect of TNC deficiency. RESULTS
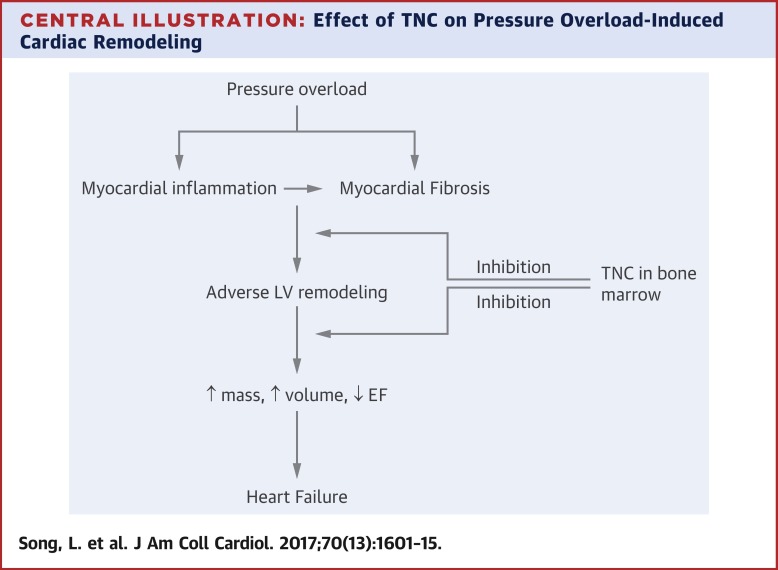
Echocardiographic analysis of pressure overloaded hearts revealed that all LV parameters (LV end-diastolic and -systolic dimensions, ejection fraction, and fractional shortening) deteriorated in TNC-deficient mice compared with their wild-type counterparts. Cardiomyocyte size and collagen accumulation were significantly greater in the absence of TNC. Mechanistically, TNC deficiency promoted rapid accumulation of the CCR2+/Ly6Chi monocyte/macrophage subset into the myocardium in response to pressure overload. Further, echocardiographic and immunohistochemical analyses of recipient hearts showed that expression of TNC in the bone marrow, but not the myocardium, protected the myocardium against excessive remodeling of the pressure-overloaded heart. CONCLUSIONS
TNC deficiency further impaired cardiac function in response to pressure overload and exacerbated fibrosis by enhancing inflammation. In addition, expression of TNC in the bone marrow, but not the myocardium, protected the myocardium against excessive remodeling in response to mild pressure overload.
中文翻译:

骨髓源性生腱蛋白-C 通过控制炎症减轻心脏肥大
背景生腱蛋白-C (TNC) 是一种高度保守的基质细胞蛋白,在发育和疾病过程中具有独特的表达模式。响应压力超载的左心室 (LV) 重塑导致胎儿基因程序的重新表达。目的 本研究的目的是研究 TNC 在心脏肥大中对压力超负荷的反应。方法 通过收缩腹主动脉或输注血管紧张素 II,在 TNC 基因敲除小鼠和野生型小鼠中诱导压力超负荷。超声心动图、免疫染色、流式细胞术、定量实时聚合酶链反应和相互骨髓移植用于评估 TNC 缺乏症的影响。结果 压力超负荷心脏的超声心动图分析显示,与野生型小鼠相比,TNC 缺陷小鼠的所有 LV 参数(LV 舒张末期和收缩末期尺寸、射血分数和缩短分数)均恶化。在没有 TNC 的情况下,心肌细胞大小和胶原蛋白积累显着增加。从机制上讲,TNC 缺乏促进了 CCR2+/Ly6Chi 单核细胞/巨噬细胞亚群在压力过载时迅速积累到心肌中。此外,受者心脏的超声心动图和免疫组织化学分析表明,TNC 在骨髓而非心肌中的表达可保护心肌免受压力过载心脏的过度重塑。结论 TNC 缺乏会进一步损害心脏功能以应对压力超负荷,并通过增强炎症加剧纤维化。此外,TNC 在骨髓而非心肌中的表达可保护心肌免受轻度压力超负荷引起的过度重塑。
更新日期:2017-09-01
中文翻译:

骨髓源性生腱蛋白-C 通过控制炎症减轻心脏肥大
背景生腱蛋白-C (TNC) 是一种高度保守的基质细胞蛋白,在发育和疾病过程中具有独特的表达模式。响应压力超载的左心室 (LV) 重塑导致胎儿基因程序的重新表达。目的 本研究的目的是研究 TNC 在心脏肥大中对压力超负荷的反应。方法 通过收缩腹主动脉或输注血管紧张素 II,在 TNC 基因敲除小鼠和野生型小鼠中诱导压力超负荷。超声心动图、免疫染色、流式细胞术、定量实时聚合酶链反应和相互骨髓移植用于评估 TNC 缺乏症的影响。结果 压力超负荷心脏的超声心动图分析显示,与野生型小鼠相比,TNC 缺陷小鼠的所有 LV 参数(LV 舒张末期和收缩末期尺寸、射血分数和缩短分数)均恶化。在没有 TNC 的情况下,心肌细胞大小和胶原蛋白积累显着增加。从机制上讲,TNC 缺乏促进了 CCR2+/Ly6Chi 单核细胞/巨噬细胞亚群在压力过载时迅速积累到心肌中。此外,受者心脏的超声心动图和免疫组织化学分析表明,TNC 在骨髓而非心肌中的表达可保护心肌免受压力过载心脏的过度重塑。结论 TNC 缺乏会进一步损害心脏功能以应对压力超负荷,并通过增强炎症加剧纤维化。此外,TNC 在骨髓而非心肌中的表达可保护心肌免受轻度压力超负荷引起的过度重塑。



























 京公网安备 11010802027423号
京公网安备 11010802027423号