当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Meta-Selective C−H Borylation of Benzylamine, Phenethylamine and Phenylpropylamine-Derived Amides Enabled by a Single Anionic Ligand
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-09-19 07:40:40 , DOI: 10.1002/anie.201708967 Holly J. Davis 1 , Georgi R. Genov 1 , Robert J. Phipps 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-09-19 07:40:40 , DOI: 10.1002/anie.201708967 Holly J. Davis 1 , Georgi R. Genov 1 , Robert J. Phipps 1
Affiliation
|
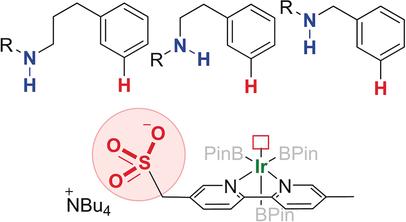
Clever positioning: A bipyridine ligand incorporating a remote anionic sulfonate group directs iridium-catalyzed borylation to the meta-position on a range of amide-containing arenes. It is proposed that this selectivity is a result of a hydrogen bonding interaction to correctly position the iridium metal centre in the crucial C−H activation.
中文翻译:

由单一阴离子配体实现的苄胺,苯乙胺和苯丙胺衍生的酰胺的亚选择性CH硼化
巧妙的定位:结合有远端阴离子磺酸盐基团的联吡啶配体可将铱催化的硼化反应引导至一系列含酰胺芳烃的间位。提出该选择性是氢键相互作用的结果,该氢键相互作用将铱金属中心正确定位在关键的CH活化中。
更新日期:2017-09-19
中文翻译:

由单一阴离子配体实现的苄胺,苯乙胺和苯丙胺衍生的酰胺的亚选择性CH硼化
巧妙的定位:结合有远端阴离子磺酸盐基团的联吡啶配体可将铱催化的硼化反应引导至一系列含酰胺芳烃的间位。提出该选择性是氢键相互作用的结果,该氢键相互作用将铱金属中心正确定位在关键的CH活化中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号