当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transition-Metal-Free Hydrogenation of Aryl Halides: From Alcohol to Aldehyde
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.orglett.7b02399 Hong-Xing Zheng 1 , Xiang-Huan Shan 1 , Jian-Ping Qu 2 , Yan-Biao Kang 1
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.orglett.7b02399 Hong-Xing Zheng 1 , Xiang-Huan Shan 1 , Jian-Ping Qu 2 , Yan-Biao Kang 1
Affiliation
|
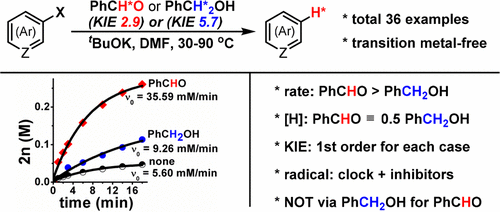
A transition-metal- and catalyst-free hydrogenation of aryl halides, promoted by bases with either aldehydes or alcohols, is described. One equivalent of benzaldehyde affords an equal yield as that of 0.5 equiv of benzyl alcohol. The kinetic study reveals that the initial rate of PhCHO is much faster than that of BnOH, in the ratio of nearly 4:1. The radical trapping experiments indicate the radical nature of this reaction. Based on the kinetic study, trapping and KIE experiments, and control experiments, a tentative mechanism is proposed. As a consequence, a wide range of (hetero)aryl iodides and bromides were efficiently reduced to their corresponding (hetero)arenes. Thus, for the first time, aldehydes are directly used as hydrogen source instead of other well-established alcohol–hydrogen sources.
中文翻译:

芳基卤化物的无过渡金属加氢:从醇到醛
描述了由碱与醛或醇一起促进的无过渡金属和无催化剂的芳基卤化物的氢化。一当量的苯甲醛可提供与0.5当量的苄醇相同的产率。动力学研究表明,PhCHO的起始速率比BnOH的起始速率快得多,比率接近4:1。自由基捕获实验表明该反应的自由基性质。在动力学研究,诱捕和KIE实验以及控制实验的基础上,提出了一种初步的机理。结果,各种(杂)芳基碘化物和溴化物被有效地还原成它们相应的(杂)芳烃。因此,醛首次被直接用作氢源,而不是其他公认的醇氢源。
更新日期:2017-09-19
中文翻译:

芳基卤化物的无过渡金属加氢:从醇到醛
描述了由碱与醛或醇一起促进的无过渡金属和无催化剂的芳基卤化物的氢化。一当量的苯甲醛可提供与0.5当量的苄醇相同的产率。动力学研究表明,PhCHO的起始速率比BnOH的起始速率快得多,比率接近4:1。自由基捕获实验表明该反应的自由基性质。在动力学研究,诱捕和KIE实验以及控制实验的基础上,提出了一种初步的机理。结果,各种(杂)芳基碘化物和溴化物被有效地还原成它们相应的(杂)芳烃。因此,醛首次被直接用作氢源,而不是其他公认的醇氢源。


























 京公网安备 11010802027423号
京公网安备 11010802027423号