当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Stereoselective Approach toward (−)-Lepadins A–C
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.orglett.7b02647 Xiong Li 1 , Lingling Hu 1 , Junhao Jia 1 , He Gu 1 , Yuanliang Jia 1 , Xiaochuan Chen 1
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.orglett.7b02647 Xiong Li 1 , Lingling Hu 1 , Junhao Jia 1 , He Gu 1 , Yuanliang Jia 1 , Xiaochuan Chen 1
Affiliation
|
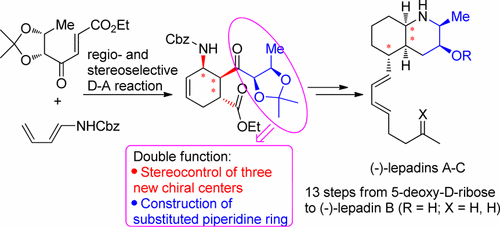
A new short approach to (−)-lepadins A–C has been developed based on a stereocontrolled Diels–Alder reaction employing a chiral dienophile. With this approach, (−)-lepadin B is synthesized from 5-deoxy-d-ribose in 13 steps with 14.8% overall yield. The cis-decahydroquinoline core containing five stereocenters could be rapidly constructed via stereoselective cycloaddition and subsequent five-step one-pot hydrogenation–cyclization.
中文翻译:

对(-)-Lepadins A–C的立体选择方法
基于使用手性亲二烯体的立体控制狄尔斯-阿尔德反应,已经开发了一种新的(-)-lepadins A-C短途径。用这种方法,( - ) - lepadin B从合成5-脱氧d -核糖在13个步骤以14.8%的总收率。可以通过立体选择性环加成反应和随后的五步一锅加氢-环化反应快速构建包含五个立体中心的顺式-十氢喹啉核心。
更新日期:2017-09-19
中文翻译:

对(-)-Lepadins A–C的立体选择方法
基于使用手性亲二烯体的立体控制狄尔斯-阿尔德反应,已经开发了一种新的(-)-lepadins A-C短途径。用这种方法,( - ) - lepadin B从合成5-脱氧d -核糖在13个步骤以14.8%的总收率。可以通过立体选择性环加成反应和随后的五步一锅加氢-环化反应快速构建包含五个立体中心的顺式-十氢喹啉核心。



























 京公网安备 11010802027423号
京公网安备 11010802027423号