Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.bmcl.2017.09.039 Aurélie Paulen , Françoise. Hoegy , Béatrice. Roche , Isabelle J. Schalk , Gaëtan L.A. Mislin
|
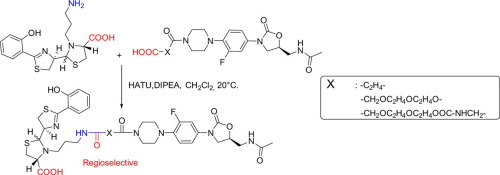
Pseudomonas aeruginosa is a Gram-negative pathogenic bacterium responsible for severe infections, and it is naturally resistant to many clinically approved antibiotic families. Oxazolidinone antibiotics are active against many Gram-positive bacteria, but are inactive against P. aeruginosa. Increasing the uptake of oxazolidinones through the bacterial envelope could lead to an increased antibiotic effect. Pyochelin is a siderophore of P. aeruginosa which delivers external iron to the bacterial cytoplasm and is a potential vector for the development of Trojan Horse oxazolidinone conjugates. Novel pyochelin-oxazolidinone conjugates were synthesized using an unexpectedly regioselective peptide coupling between an amine functionalized pyochelin and oxazolidinones functionalized with a terminal carboxylate.
中文翻译:

恶唑烷酮类抗生素与pyyochelin类似物偶联物的合成
铜绿假单胞菌是引起严重感染的革兰氏阴性致病菌,对许多临床批准的抗生素家族具有天然抗性。恶唑烷酮抗生素对许多革兰氏阳性细菌有活性,但对铜绿假单胞菌则无活性。通过细菌包膜增加对恶唑烷酮的吸收可导致增强的抗生素作用。pychelin是铜绿假单胞菌的铁载体它可以将外部铁输送到细菌细胞质中,并且是开发特洛伊木马恶唑烷酮共轭物的潜在载体。使用胺官能化的秋水仙素和末端羧酸盐官能化的恶唑烷酮之间的意外区域选择性肽偶联,合成了新型的pyochelin-恶唑烷酮共轭物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号