Catalysis Today ( IF 5.3 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.cattod.2017.09.030 No-Kuk Park , Yong Han Jeong , Jin Wook Lee , Tae Jin Lee
|
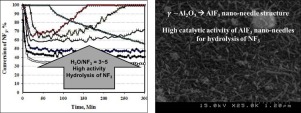
This study examined the catalytic properties of AlF3 nanostructures for the hydrolysis of NF3 discharged from semiconductor processes. Alumina being used as a catalyst for the hydrolysis of NF3 was converted to AlF3 by fluorination in the presence of NF3 during a gas-solid chemical reaction. In general, AlF3 has low catalytic activity in the hydrolysis of fluorinated compounds, but its catalytic activity varies according to its crystal structure. Commercial AlF3 exhibited low catalytic activity in the hydrolysis of NF3, whereas AlF3 formed from the chemical reaction between NF3 and alumina exhibited very high catalytic activity. Although alumina has high catalytic activity in the hydrolysis of NF3, its activity decreases as it is converted to AlF3 during the process. On the other hand, the activity test and X-ray diffraction showed that its catalytic activity is recovered as the nanocrystals of AlF3 were grown. AlF3 nanocrystals grown by the gas-solid chemical reaction showed a similar distribution of acid sites as the alumina. These acid sites provided the catalytic activity of AlF3 in the hydrolysis of NF3.
中文翻译:

AlF 3纳米结构对NF 3水解的催化活性
这项研究检查了AlF 3纳米结构对从半导体工艺排出的NF 3的水解的催化性能。在气固化学反应期间,在NF 3的存在下,通过氟化将用作NF 3水解催化剂的氧化铝转化为AlF 3。通常,AlF 3在氟化化合物的水解中具有低催化活性,但是其催化活性根据其晶体结构而变化。商业化的AlF 3在NF 3的水解中表现出较低的催化活性,而AlF 3是由NF 3之间的化学反应形成的氧化铝具有很高的催化活性。尽管氧化铝在NF 3的水解中具有很高的催化活性,但由于在该过程中转化为AlF 3,其活性会降低。另一方面,活性测试和X射线衍射表明,随着AlF 3纳米晶体的生长,其催化活性得以恢复。通过气固化学反应生长的AlF 3纳米晶体显示出与氧化铝相似的酸位分布。这些酸位提供了AlF 3在NF 3水解中的催化活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号