当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process
Water Research ( IF 12.8 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.watres.2017.09.028 Zihao Wu , Kaiheng Guo , Jingyun Fang , Xueqin Yang , Hong Xiao , Shaodong Hou , Xiujuan Kong , Chii Shang , Xin Yang , Fangang Meng , Liwei Chen
Water Research ( IF 12.8 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.watres.2017.09.028 Zihao Wu , Kaiheng Guo , Jingyun Fang , Xueqin Yang , Hong Xiao , Shaodong Hou , Xiujuan Kong , Chii Shang , Xin Yang , Fangang Meng , Liwei Chen
|
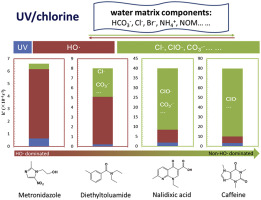
The UV/chlorine process is an emerging advanced oxidation process (AOP) that produces various reactive species, such as hydroxyl radicals (HO) and reactive chlorine species (RCS). The effects of the treatment conditions, such as chlorine dosage and pH, and the water matrix components of natural organic matter (NOM), alkalinity, ammonia and halides, on the kinetics and reactive species in the degradation of four micropollutants, metronidazole (MDZ), nalidixic acid (NDA), diethyltoluamide (DEET) and caffeine (CAF), by the UV/chlorine process were investigated. The degradation of MDZ and CAF was primarily attributable to HO and ClO
and ClO , respectively, while that of NDA was primarily attributable to both ClO
, respectively, while that of NDA was primarily attributable to both ClO and CO3
and CO3 -. HO
-. HO , Cl
, Cl and CO3
and CO3 - are important for the degradation of DEET. The second-order rate constants for ClO
- are important for the degradation of DEET. The second-order rate constants for ClO with CAF and CO3
with CAF and CO3 - with NDA were determined to be 5.1 (±0.2) × 107 M−1s−1 and 1.4 (±0.1) × 107 M−1s−1, respectively. Increasing chlorine dosage slightly changed the contribution of HO
- with NDA were determined to be 5.1 (±0.2) × 107 M−1s−1 and 1.4 (±0.1) × 107 M−1s−1, respectively. Increasing chlorine dosage slightly changed the contribution of HO but linearly increased that of ClO
but linearly increased that of ClO to micropollutant degradation. Increasing pH decreased the contribution of either HO
to micropollutant degradation. Increasing pH decreased the contribution of either HO or Cl
or Cl but not that of ClO
but not that of ClO . Both NOM and bicarbonate decreased the contributions of HO
. Both NOM and bicarbonate decreased the contributions of HO and Cl
and Cl , whereas NOM but not bicarbonate significantly decreased that of ClO
, whereas NOM but not bicarbonate significantly decreased that of ClO . The contribution of either HO
. The contribution of either HO or Cl
or Cl first rose and then fell as the molar ratio of ammonia to chlorine increased from 0 to 1:1, while that of ClO
first rose and then fell as the molar ratio of ammonia to chlorine increased from 0 to 1:1, while that of ClO decreased. The co-presence of high concentrations of Cl− and Br− enhanced the contribution of ClBr
decreased. The co-presence of high concentrations of Cl− and Br− enhanced the contribution of ClBr - and BrCl.
- and BrCl.
中文翻译:

影响反应物种在UV /氯过程中降解微污染物的作用的因素
UV /氯气工艺是一种新兴的高级氧化工艺(AOP),可产生各种反应性物质,例如羟基自由基(HO)和活性氯(RCS)。氯含量和pH值,天然有机物(NOM)的水基质成分,碱度,氨和卤化物等处理条件对四种微量污染物甲硝唑(MDZ)降解动力学和反应物种的影响分别通过紫外线/氯气工艺对萘啶酸(NDA),二乙基甲苯酰胺(DEET)和咖啡因(CAF)进行了研究。MDZ和CAF的降解主要归因于HO 和ClO
和ClO 分别为NDA的主要归因于两个ClO
分别为NDA的主要归因于两个ClO 和CO 3
和CO 3 -。HO
-。HO ,氯
,氯 和CO 3
和CO 3 -对于DEET的降解很重要。ClO的二阶速率常数
-对于DEET的降解很重要。ClO的二阶速率常数 带有CAF和CO 3
带有CAF和CO 3 -具有NDA的-分别被确定为5.1(±0.2)×10 7 M -1 s -1和1.4(±0.1)×10 7 M -1 s -1。增加氯剂量会稍微改变HO的贡献
-具有NDA的-分别被确定为5.1(±0.2)×10 7 M -1 s -1和1.4(±0.1)×10 7 M -1 s -1。增加氯剂量会稍微改变HO的贡献 但线性增加了ClO
但线性增加了ClO 对微污染物的降解。pH值升高会降低HO的贡献
对微污染物的降解。pH值升高会降低HO的贡献 或Cl
或Cl 但不是ClO
但不是ClO 。NOM和碳酸氢盐均降低了HO的贡献
。NOM和碳酸氢盐均降低了HO的贡献 和Cl
和Cl ,而NOM(而非碳酸氢盐)显着降低了ClO的浓度
,而NOM(而非碳酸氢盐)显着降低了ClO的浓度 。任何的贡献
。任何的贡献 或Cl
或Cl 随着氨与氯的摩尔比从0增加到1:1,ClO的浓度先上升然后下降
随着氨与氯的摩尔比从0增加到1:1,ClO的浓度先上升然后下降 减少了。高浓度的Cl的共同存在-和Br -增强CLBR的贡献
减少了。高浓度的Cl的共同存在-和Br -增强CLBR的贡献 -和BrCl。
-和BrCl。
更新日期:2017-09-19
 and ClO
and ClO , respectively, while that of NDA was primarily attributable to both ClO
, respectively, while that of NDA was primarily attributable to both ClO and CO3
and CO3 -. HO
-. HO , Cl
, Cl and CO3
and CO3 - are important for the degradation of DEET. The second-order rate constants for ClO
- are important for the degradation of DEET. The second-order rate constants for ClO with CAF and CO3
with CAF and CO3 - with NDA were determined to be 5.1 (±0.2) × 107 M−1s−1 and 1.4 (±0.1) × 107 M−1s−1, respectively. Increasing chlorine dosage slightly changed the contribution of HO
- with NDA were determined to be 5.1 (±0.2) × 107 M−1s−1 and 1.4 (±0.1) × 107 M−1s−1, respectively. Increasing chlorine dosage slightly changed the contribution of HO but linearly increased that of ClO
but linearly increased that of ClO to micropollutant degradation. Increasing pH decreased the contribution of either HO
to micropollutant degradation. Increasing pH decreased the contribution of either HO or Cl
or Cl but not that of ClO
but not that of ClO . Both NOM and bicarbonate decreased the contributions of HO
. Both NOM and bicarbonate decreased the contributions of HO and Cl
and Cl , whereas NOM but not bicarbonate significantly decreased that of ClO
, whereas NOM but not bicarbonate significantly decreased that of ClO . The contribution of either HO
. The contribution of either HO or Cl
or Cl first rose and then fell as the molar ratio of ammonia to chlorine increased from 0 to 1:1, while that of ClO
first rose and then fell as the molar ratio of ammonia to chlorine increased from 0 to 1:1, while that of ClO decreased. The co-presence of high concentrations of Cl− and Br− enhanced the contribution of ClBr
decreased. The co-presence of high concentrations of Cl− and Br− enhanced the contribution of ClBr - and BrCl.
- and BrCl.
中文翻译:

影响反应物种在UV /氯过程中降解微污染物的作用的因素
UV /氯气工艺是一种新兴的高级氧化工艺(AOP),可产生各种反应性物质,例如羟基自由基(HO)和活性氯(RCS)。氯含量和pH值,天然有机物(NOM)的水基质成分,碱度,氨和卤化物等处理条件对四种微量污染物甲硝唑(MDZ)降解动力学和反应物种的影响分别通过紫外线/氯气工艺对萘啶酸(NDA),二乙基甲苯酰胺(DEET)和咖啡因(CAF)进行了研究。MDZ和CAF的降解主要归因于HO
 和ClO
和ClO 分别为NDA的主要归因于两个ClO
分别为NDA的主要归因于两个ClO 和CO 3
和CO 3 -。HO
-。HO ,氯
,氯 和CO 3
和CO 3 -对于DEET的降解很重要。ClO的二阶速率常数
-对于DEET的降解很重要。ClO的二阶速率常数 带有CAF和CO 3
带有CAF和CO 3 -具有NDA的-分别被确定为5.1(±0.2)×10 7 M -1 s -1和1.4(±0.1)×10 7 M -1 s -1。增加氯剂量会稍微改变HO的贡献
-具有NDA的-分别被确定为5.1(±0.2)×10 7 M -1 s -1和1.4(±0.1)×10 7 M -1 s -1。增加氯剂量会稍微改变HO的贡献 但线性增加了ClO
但线性增加了ClO 对微污染物的降解。pH值升高会降低HO的贡献
对微污染物的降解。pH值升高会降低HO的贡献 或Cl
或Cl 但不是ClO
但不是ClO 。NOM和碳酸氢盐均降低了HO的贡献
。NOM和碳酸氢盐均降低了HO的贡献 和Cl
和Cl ,而NOM(而非碳酸氢盐)显着降低了ClO的浓度
,而NOM(而非碳酸氢盐)显着降低了ClO的浓度 。任何的贡献
。任何的贡献 或Cl
或Cl 随着氨与氯的摩尔比从0增加到1:1,ClO的浓度先上升然后下降
随着氨与氯的摩尔比从0增加到1:1,ClO的浓度先上升然后下降 减少了。高浓度的Cl的共同存在-和Br -增强CLBR的贡献
减少了。高浓度的Cl的共同存在-和Br -增强CLBR的贡献 -和BrCl。
-和BrCl。



























 京公网安备 11010802027423号
京公网安备 11010802027423号