当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diasteroselective and Enantioselective Ir-Catalyzed Allylic Substitutions of 1-Substituted 1-Fluoro-1-(arenesulfonyl)methylene Derivatives
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.joc.7b01782 Jiteng Chen 1, 2 , Xiaoming Zhao 1, 2 , Wenyan Dan 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acs.joc.7b01782 Jiteng Chen 1, 2 , Xiaoming Zhao 1, 2 , Wenyan Dan 1, 2
Affiliation
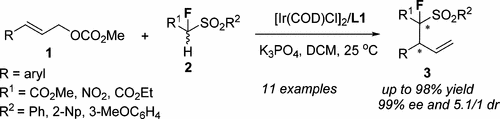
|
diasteroselective and enantioselective Ir-catalyzed allylic substitutions of 1-substituted 1-fluoro-1-(arenesulfonyl)methylene derivatives are presented, which afford the fluorinated allyl products with two chirality centers. The steric demand of 1-substituted 1-fluoro-1-(arenesulfonyl)methylene derivatives and allylic substrates has a great influence on the dr values of these reactions. The transformation of the branched allyl product into the fluorinated 3,4-dihydro-2H-pyrrole 1-oxide was discussed, as well.
中文翻译:

1-取代的1-氟-1-(芳烃磺酰基)亚甲基衍生物的非对映选择性和对映选择性Ir催化的烯丙基取代
提出了1-取代的1-氟-1-(芳烃磺酰基)亚甲基衍生物的非对映选择性和对映选择性Ir催化的烯丙基取代,其提供了具有两个手性中心的氟化烯丙基产物。1-取代的1-氟-1-(芳烃磺酰基)亚甲基衍生物和烯丙基底物的空间需求对这些反应的dr值有很大影响。还讨论了支链烯丙基产物向氟化的3,4-二氢-2 H-吡咯1-氧化物的转化。
更新日期:2017-09-19
中文翻译:

1-取代的1-氟-1-(芳烃磺酰基)亚甲基衍生物的非对映选择性和对映选择性Ir催化的烯丙基取代
提出了1-取代的1-氟-1-(芳烃磺酰基)亚甲基衍生物的非对映选择性和对映选择性Ir催化的烯丙基取代,其提供了具有两个手性中心的氟化烯丙基产物。1-取代的1-氟-1-(芳烃磺酰基)亚甲基衍生物和烯丙基底物的空间需求对这些反应的dr值有很大影响。还讨论了支链烯丙基产物向氟化的3,4-二氢-2 H-吡咯1-氧化物的转化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号