当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantification and Theoretical Analysis of the Electrophilicities of Michael Acceptors
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-09-18 , DOI: 10.1021/jacs.7b05106 Dominik S. Allgäuer 1 , Harish Jangra 1 , Haruyasu Asahara 1 , Zhen Li 1 , Quan Chen 1 , Hendrik Zipse 1 , Armin R. Ofial 1 , Herbert Mayr 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-09-18 , DOI: 10.1021/jacs.7b05106 Dominik S. Allgäuer 1 , Harish Jangra 1 , Haruyasu Asahara 1 , Zhen Li 1 , Quan Chen 1 , Hendrik Zipse 1 , Armin R. Ofial 1 , Herbert Mayr 1
Affiliation
|
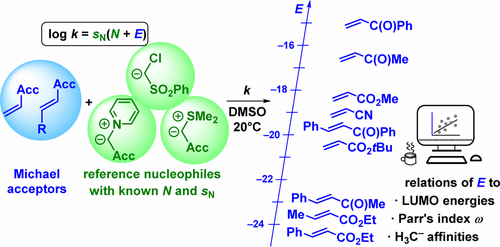
In order to quantify the electrophilic reactivities of common Michael acceptors, we measured the kinetics of the reactions of monoacceptor-substituted ethylenes (H2C═CH-Acc, 1) and styrenes (PhCH═CH-Acc, 2) with pyridinium ylides 3, sulfonium ylide 4, and sulfonyl-substituted chloromethyl anion 5. Substitution of the 57 measured second-order rate constants (log k) and the previously reported nucleophile-specific parameters N and sN for 3-5 into the correlation log k = sN(E + N) allowed us to calculate 15 new empirical electrophilicity parameters E for Michael acceptors 1 and 2. The use of the same parameters sN, N, and E for these different types of reactions shows that all reactions proceed via a common rate-determining step, the nucleophilic attack of 3-5 at the Michael acceptors with formation of acyclic intermediates, which subsequently cyclize to give tetrahydroindolizines (stepwise 1,3-dipolar cycloadditions with 3) and cyclopropanes (with 4 and 5), respectively. The electrophilicity parameters E thus determined can be used to calculate the rates of the reactions of Michael acceptors 1 and 2 with any nucleophile of known N and sN. DFT calculations were performed to confirm the suggested reaction mechanisms and to elucidate the origin of the electrophilic reactivities. While electrophilicities E correlate poorly with the LUMO energies and with Parr's electrophilicity index ω, good correlations were found between the experimentally observed electrophilic reactivities of 44 Michael acceptors and their calculated methyl anion affinities, particularly when solvation by dimethyl sulfoxide was taken into account by applying the SMD continuum solvation model. Because of the large structural variety of Michael acceptors considered for these correlations, which cover a reactivity range of 17 orders of magnitude, we consider the calculation of methyl anion affinities to be the method of choice for a rapid estimate of electrophilic reactivities.
中文翻译:

迈克尔受体亲电性的量化和理论分析
随后分别环化生成四氢茚茚(与 3 的逐步 1,3-偶极环加成)和环丙烷(与 4 和 5)。由此确定的亲电性参数 E 可用于计算迈克尔受体 1 和 2 与任何已知 N 和 sN 的亲核试剂的反应速率。进行 DFT 计算以确认建议的反应机制并阐明亲电反应性的起源。虽然亲电性 E 与 LUMO 能量和帕尔的亲电性指数 ω 相关性较差,但在实验观察到的 44 个迈克尔受体的亲电反应性与其计算的甲基阴离子亲和力之间发现了良好的相关性,特别是当通过应用考虑二甲基亚砜的溶剂化时SMD 连续溶剂化模型。
更新日期:2017-09-18
中文翻译:

迈克尔受体亲电性的量化和理论分析
随后分别环化生成四氢茚茚(与 3 的逐步 1,3-偶极环加成)和环丙烷(与 4 和 5)。由此确定的亲电性参数 E 可用于计算迈克尔受体 1 和 2 与任何已知 N 和 sN 的亲核试剂的反应速率。进行 DFT 计算以确认建议的反应机制并阐明亲电反应性的起源。虽然亲电性 E 与 LUMO 能量和帕尔的亲电性指数 ω 相关性较差,但在实验观察到的 44 个迈克尔受体的亲电反应性与其计算的甲基阴离子亲和力之间发现了良好的相关性,特别是当通过应用考虑二甲基亚砜的溶剂化时SMD 连续溶剂化模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号