European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.ejmech.2017.09.025 Yi Zou , Fang Wang , Yan Wang , Qirui Sun , Yue Hu , Yuezhen Li , Wen Liu , Wenjie Guo , Zhangjian Huang , Yihua Zhang , Qiang Xu , Yisheng Lai
|
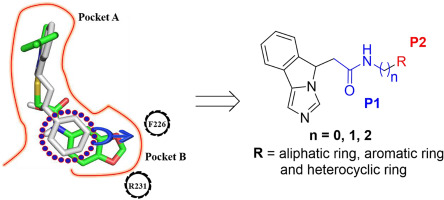
Indoleamine-2,3-dioxygenase-1 (IDO1) is an attractive target for cancer immunotherapy. Herein, a series of novel imidazoleisoindole derivatives were prepared and evaluated for their ability to inhibit IDO1. Among these, derivative 11r was the most active compound with nanomolar potency in the Hela cell-based assay, while showed negligible cellular toxicity. UV-visible spectra study demonstrated that compounds 11p and 11r bound to IDO1 and coordinated with the heme iron. Furthermore, they could significantly promote T cell proliferation, increase IFN-γ production, and reduce the numbers of Foxp3+ regulatory T cells. Finally, induced fit docking (IFD) and quantum mechanics/molecular mechanics (QM/MM) calculation were performed to understand the interactions of these compounds to IDO1 protein, which provided a comprehensive guide for further structural modification and optimization.
中文翻译:

咪唑异吲哚衍生物作为有效IDO1抑制剂的发现:设计,合成,生物学评估和计算研究
吲哚胺-2,3-二加氧酶-1(IDO1)是癌症免疫治疗的诱人靶标。本文中,制备了一系列新型咪唑异吲哚衍生物,并评估了它们抑制IDO1的能力。其中,在基于Hela细胞的测定中,衍生物11r是最具活性的纳摩尔浓度化合物,而细胞毒性却可以忽略不计。紫外可见光谱研究表明,化合物11p和11r与IDO1结合并与血红素铁配位。此外,它们可以显着促进T细胞增殖,增加IFN- γ的产生并减少Foxp3 +的数量。调节性T细胞。最后,进行了诱导拟合对接(IFD)和量子力学/分子力学(QM / MM)计算,以了解这些化合物与IDO1蛋白的相互作用,为进一步的结构修饰和优化提供了全面的指南。


























 京公网安备 11010802027423号
京公网安备 11010802027423号