European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.ejmech.2017.09.022 Fabiana Pandolfi , Daniela De Vita , Martina Bortolami , Antonio Coluccia , Roberto Di Santo , Roberta Costi , Vincenza Andrisano , Francesco Alabiso , Christian Bergamini , Romana Fato , Manuela Bartolini , Luigi Scipione
|
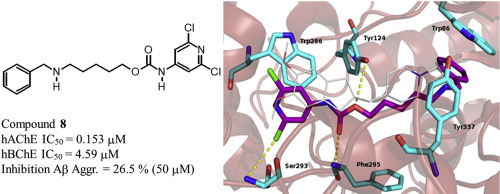
A new series of pyridine derivatives with carbamic or amidic function has been designed and synthesized to act as cholinesterase inhibitors. The synthesized compounds were tested toward EeAChE and hAChE and toward eqBChE and hBChE. The carbamate 8 was the most potent hAChE inhibitor (IC50 = 0.153 ± 0.016 μM) while the carbamate 11 was the most potent inhibitor of hBChE (IC50 = 0.828 ± 0.067 μM). A molecular docking study indicated that the carbamate 8 was able to bind AChE by interacting with both CAS and PAS, in agreement with the mixed inhibition mechanism. Furthermore, the carbamates 8, 9 and 11 were able to inhibit Aβ42 self-aggregation and possessed quite low toxicity against human astrocytoma T67 and HeLa cell lines, being the carbamate 8 the less toxic compound on both cell lines.
中文翻译:

新的吡啶衍生物作为乙酰胆碱酯酶和淀粉样蛋白聚集的抑制剂
已经设计并合成了一系列具有氨基甲酸酯或酰胺功能的吡啶衍生物,以用作胆碱酯酶抑制剂。测试了合成化合物的抗Ee AChE和h AChE以及eq BChE和h BChE。氨基甲酸酯8是最有效的h AChE抑制剂(IC 50 = 0.153±0.016μM),而氨基甲酸酯11是最有效的h BChE抑制剂(IC 50 = 0.828±0.067μM)。分子对接研究表明,氨基甲酸酯8通过与CAS和PAS相互作用,能够与AChE结合,这与混合抑制机制一致。此外,氨基甲酸酯8,9和11能够抑制Aβ 42自聚集和具有针对人星形细胞瘤T67和HeLa细胞系中相当低的毒性,作为氨基甲酸酯8上这两种细胞系的毒性的化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号